Abstract
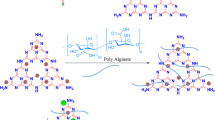
Herein, bio-based alginates (Alg) containing metallic beads (Ce and Cu) were synthesized via an alginate cross-linking method, and their properties were studied using experimental techniques combined with theoretical simulations. Materials were characterized through Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and scanning electron microscope (SEM) images, to determine the cross-linking structural features, thermal stability, and surface morphology of alginates. Besides, density functional theory (DFT) methods were employed to calculate global reactivity parameters such as HOMO–LUMO gap energies (ΔEH-L), electronegativity (χ), hardness (η), and electrophilic and nucleophilic indicators, using both gas and aqueous media for the study of the complexation process. Among other features, characterization of the thermal properties showed that Alg@Ce and Alg@Cu alginate beads behave differently as a function of the temperature. This behavior was also predicted by the conformation energy differences between Alg@Ce and Alg@Cu, which were found out theoretically and explained with the combined study of the vibrational modes between the carboxylate group with either Ce or Cu. Overall, the reactivity of the Alg@Ce alginate bead was higher than that of the Alg@Cu counterpart, results could be used as a cornerstone to employed the materials here studied in a wide range of applications.
Graphical abstract










Similar content being viewed by others

Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
All data underlying the results are available as part of the article and no additional source data are required. Software code and availability from https://gaussian.com/.
References
Abdellaoui Y, Celaya CA, Elhoudi M et al (2022) Understanding of vibrational and thermal behavior of bio-based doped alginate@nickel cross-linked beads: a combined experimental and theoretical study. J Mol Struct 1249:131524. https://doi.org/10.1016/j.molstruc.2021.131524
Abou Oualid H, Essamlali Y, Amadine O et al (2019) Green synthesis of Ag/ZnO nanohybrid using sodium alginate gelation method. RSC Adv 43:13786–13790
Abou Oualid H, Abdellaoui Y, Laabd M et al (2020) Eco-efficient green seaweed codium decorticatum biosorbent for textile dyes : characterization, mechanism, recyclability, and RSM optimization. ACS Omega. https://doi.org/10.1021/acsomega.0c02311
Agulhon P, Markova V, Robitzer M et al (2012) Structure of alginate gels: interaction of diuronate units with divalent cations from density functional calculations. Biomacromol 13:1899–1907. https://doi.org/10.1021/bm300420z
Banerjee S, Banerjee A, Sarkar P (2018) Toxic / hazardous substances and environmental engineering statistical optimization of arsenic biosorption by microbial enzyme via Ca-alginate beads. J Environ Sci Heal Part A 4529:. https://doi.org/10.1080/10934529.2017.1409009
Barra I, Kharbach M, Bousrabat M et al (2020) Discrimination of diesel fuels marketed in Morocco using FTIR. GC-MS analysis and chemometrics methods Talanta 209:120543. https://doi.org/10.1016/j.talanta.2019.120543
Beatriz H, Peixoto T, Araújo D et al (2020) International Journal of Biological Macromolecules chitosan, alginate and other macromolecules as activated carbon immobilizing agents : a review on composite adsorbents for the removal of water contaminants. Int J Biol Macromol 164:2535–2549. https://doi.org/10.1016/j.ijbiomac.2020.08.118
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Belitz H-D, Grosch W, Schieberle P (2004) Food additives. In: Food chemistry. Springer, pp 434–473
Bourzi H, Oukhrib R, Ibrahimi B El, et al (2020) Furfural analogs as sustainable corrosion inhibitors-predictive efficiency using DFT and monte carlo simulations on the Cu(111), Fe(110), Al(111) and Sn(111) surfaces in acid media. Sustain 12:. https://doi.org/10.3390/SU12083304
Bourzi H, Oukhrib R, Ibrahimi B El, et al (2020) Understanding of anti-corrosive behavior of some tetrazole derivatives in acidic medium: adsorption on Cu (111) surface using quantum chemical calculations and Monte Carlo simulations. Surf Sci 702:. https://doi.org/10.1016/j.susc.2020.121692
Broido A, Forest PS, Station RE (1969) A simple , sensitive graphical method of treating thermogravimetric analysis data *. 1773:1761–1773
Caldeweyher E, Bannwarth C, Grimme S (2017) Extension of the D3 dispersion coefficient model. J Chem Phys 147:034112. https://doi.org/10.1063/1.4993215
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Chai LQ, Zhang XF, Tang LJ (2021) Crystallographic, spectroscopic, TD/DFT calculations and Hirshfeld surface analysis of cadmium(II) coordination polymer containing pyridine ring. J Mol Struct 1245:131028. https://doi.org/10.1016/j.molstruc.2021.131028
Cimá-Mukul CA, Abdellaoui Y, Abatal M et al (2019) Eco-Efficient biosorbent based on leucaena leucocephala residues for the simultaneous removal of Pb(II) and Cd(II) ions from water system: sorption and mechanism. Bioinorg Chem Appl 2019:1–13. https://doi.org/10.1155/2019/2814047
Chu KH (2004) Improved fixed bed models for metal biosorption. Chem Eng J 97:233–239. https://doi.org/10.1016/S1385-8947(03)00214-6
Draget KI, Taylor C (2011) Food Hydrocolloids chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll 25:251–256. https://doi.org/10.1016/j.foodhyd.2009.10.007
Draget KI Alginates. Handb Hydrocoll Work. https://doi.org/10.1533/9781845695873.807
Dennington R, Keith TA, Millam JM (2016) GaussView, version 6.0. 16. Semichem Inc Shawnee Mission KS
Elhoudi M, Aarab N, Laabd M (2021) Quantum chemical approach ( DFT ) of the binary complexation of Hg ( II ). 3:406–415
Elhoudi M, Hsini A, El Houdi M et al (2021) A theoretical investigation of complexation for pyrimidine bases with Hg2+ and Cd2+ by DFT method. Nanotechnol Environ Eng 6:1–13. https://doi.org/10.1007/s41204-021-00128-x
Fandrich R, Gu Y, Burrows D, Moeller K (2007) Modern SEM-based mineral liberation analysis. 84:310–320. https://doi.org/10.1016/j.minpro.2006.07.018
Fourest E, Volesky B (1996) Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ Sci Technol 30:277–282. https://doi.org/10.1021/es950315s
Gaussian09 RA (2009) 1, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, v. Barone, b. Mennucci, ga petersson et al., gaussian. Inc, Wallingford CT 121:150–166
Goh CH, Wan P, Heng S, Chan LW (2012) Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr Polym 88:1–12. https://doi.org/10.1016/j.carbpol.2011.11.012
Gyu H, Won T, Yun M, Yoo I (2007) Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem 42:1371–1377. https://doi.org/10.1016/j.procbio.2007.06.016
Hanwell MD, Curtis DE, Lonie DC et al (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17
Hassan B, Rajan VK, Mujeeb VMA, Muraleedharan K (2017) A DFT based analysis of adsorption of Hg2+ion on chitosan monomer and its citralidene and salicylidene derivatives: prior to the removal of Hg toxicity. Int J Biol Macromol 99:549–554. https://doi.org/10.1016/j.ijbiomac.2017.03.032
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864
Jmiai A, El Ibrahimi B, Tara A et al (2018) Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: experimental and theoretical approaches. J Mol Struct 1157:408–417. https://doi.org/10.1016/j.molstruc.2017.12.060
Kharbach M, Marmouzi I, Kamal R et al (2021) Extra virgin Argan oils’ shelf-life monitoring and prediction based on chemical properties or FTIR fingerprints and chemometrics. Food Control 121:107607. https://doi.org/10.1016/j.foodcont.2020.107607
Kikuchi A, Okano T (2002) Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev 54:53–77. https://doi.org/10.1016/S0169-409X(01)00243-5
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Larosa C, Salerno M, de Lima JS et al (2018) Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int J Biol Macromol 115:900–906. https://doi.org/10.1016/j.ijbiomac.2018.04.138
Laurienzo P (2010) Marine polysaccharides in pharmaceutical applications : an overview. Mar Drugs 8:2435–2465. https://doi.org/10.3390/md8092435
Liu Y, Zhao J-C, Zhang C-J et al (2015) Bio-based nickel alginate and copper alginate films with excellent flame retardancy: preparation, flammability and thermal degradation behavior. RSC Adv 5:64125–64137. https://doi.org/10.1039/C5RA11048C
Majidnia Z, Idris A (2014) Evaluation of cesium removal from radioactive waste water using. Chem Eng J. https://doi.org/10.1016/j.cej.2014.09.118
McGoverin CM, Walsh TJ, Gordon KC et al (2007) Predicting nonlinear optical properties in push-pull molecules based on methyl pyridinium donor and 3-cyano-5,5-dimethyl-2(5H)-furanylidene-propanedinitrile acceptor units using vibrational spectroscopy and density functional theory. Chem Phys Lett 443:298–303. https://doi.org/10.1016/j.cplett.2007.06.068
Mokhena TC, Luyt AS (2017) Electrospun alginate nanofibres impregnated with silver nanoparticles: preparation morphology and antibacterial properties. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2017.02.068
Monakhova Y, Agulhon P, Quignard F et al (2012) New mixed lanthanum- and alkaline-earth cation-containing basic catalysts obtained by an alginate route. Catal Today 189:28–34. https://doi.org/10.1016/j.cattod.2012.03.072
(1991) Book review. 47:1991
Oualid HA, Amadine O, Essamlali Y et al (2019) Highly efficient catalytic/sonocatalytic reduction of 4-nitrophenol and antibacterial activity through a bifunctional Ag/ZnO nanohybrid material prepared via a sodium alginate method. Nanoscale Adv 1:3151–3163. https://doi.org/10.1039/C9NA00075E
Oualid HA, Amadine O, Essamlali Y, et al Supercritical CO 2 drying of alginate/zinc hydrogels: a green and facile route to prepare ZnO foam structures and ZnO nanoparticles. https://doi.org/10.1039/c8ra02129e
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807. https://doi.org/10.1063/1.436185
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Parr RG, Yang W (1950) Density Functional theory of atoms and molecules.-Oxford University Press, 1989. Citado 2:19
Pavelková M, Vysloužil J, Kubová K et al (2021) Assessment of antimicrobic, antivirotic and cytotoxic potential of alginate beads cross-linked by bivalent ions for vaginal administration. Pharmaceutics 13:1–20. https://doi.org/10.3390/pharmaceutics13020165
Plazinski W (2013) Binding of heavy metals by algal biosorbents. Theoretical models of kinetics, equilibria and thermodynamics. Adv Colloid Interface Sci 197–198:58–67. https://doi.org/10.1016/j.cis.2013.04.002
Prabhu AA, Chityala S, Jayachandran D et al (2020) A two step optimization approach for maximizing biosorption of hexavalent chromium ions ( Cr ( VI )) using alginate immobilized Sargassum sp in a packed bed column. Sep Sci Technol 00:1–17. https://doi.org/10.1080/01496395.2019.1708933
Quignard F, Valentin R, Di Renzo F (2008) Aerogel materials from marine polysaccharides. New J Chem 32:1300–1310. https://doi.org/10.1039/b808218a
RachidOukhriba Hicham Abou Oualid, Youness Abdellaoui, Souad El Issami, LahcenBazzi, Mustapha Hilali, Hassan Bourzi BEI (2020) In silico investigations of alginate biopolymer on the Fe (110), Cu (111), Al (111) and Sn (001) surfaces in acidic media: Quantum chemical and molecular mechanic calculations. J Mol Liq
Resmi R, Parvathy J, John A, Joseph R (2020) Injectable self-crosslinking hydrogels for meniscal repair : a study with oxidized alginate and gelatin. Carbohydr Polym 234:115902. https://doi.org/10.1016/j.carbpol.2020.115902
Rubab S, Anwaar M, Ekinci D et al (2020) International Journal of Biological Macromolecules Multifunctional alginate-based hydrogel with reversible crosslinking for controlled therapeutics delivery. Int J Biol Macromol 150:315–325. https://doi.org/10.1016/j.ijbiomac.2020.02.042
Seal P, Jha PC, Chakrabarti S (2008) Static first order hyperpolarizabilities of DNA base pairs: a configuration interaction study. J Mol Struct THEOCHEM 855:64–68. https://doi.org/10.1016/j.theochem.2008.01.002
Sprio S, Sandri M, Panseri S, et al (2014) Bone substitutes based on biomineralization. Woodhead Publishing Limited
Varaprasad K, Jayaramudu T, Kanikireddy V et al (2020) Alginate-based composite materials for wound dressing application : a mini review. Carbohydr Polym 236:116025. https://doi.org/10.1016/j.carbpol.2020.116025
Winkleman A, Bracher PJ, Gitlin I, Whitesides GM (2007) Fabrication and manipulation of ionotropic hydrogels cross-linked by paramagnetic ions. Chem Mater 19:1362–1368. https://doi.org/10.1021/cm062626f
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Contributions
Conceptualization: formulation and evolution of overarching research goals an aims: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya, Youness Abdellaoui, and Hicham Abou Oualid.
Data curation: produce data, including software code use: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya, Miguel Reina.
Investigation: performing the experiments: Youness Abdellaoui, Issam Barra, Younes Brahmi, Hassan Bourzi.
Formal analysis: analyze study data: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya, Youness Abdellaoui, Issam Barra, Younes Brahmi, Hassan Bourzi.
Project administration: management and coordination responsibility for the research activity planning and execution:Youness Abdellaoui, Hicham Abou Oualid.
Resources: provision of study materials, reagents, laboratory simples, instrumentation computing resources; other analysis tools: Christian A. Celaya, Youness Abdellaoui, Issam Barra, Younes Brahmi, Hassan Bourzi.
Software: implementation of the computer code and supporting algorithms: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya.
Supervision: oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team: Youness Abdellaoui, Abdallah Albourine, Hicham Abou Oualid.
Validation: verification, whether as a part of the activity or separate, of the overall replication or reproductivity of results/experiments and other research outputs: Daniel G. Araiza, Youness Abdellaoui, Miguel Reina, Hicham Abou Oualid.
Visualization: preparation, creation and/or presentation of the published work, specifically visualization/data presentation: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya, Youness Abdellaoui, Hicham Abou Oualid.
Writing-original draft: preparation, creation and/or presentation of the published work, specifically writing the initial draft: Mohammed Elhoudi, Rachid Oukhrib, Christian A. Celaya, Daniel G. Araiza.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammed Elhoudi and Rachid Oukhrib contributed equally to this work.
Rights and permissions
About this article
Cite this article
Elhoudi, M., Oukhrib, R., A. Celaya, C. et al. Comparison of green bio-based cerium/alginate vs. copper/alginate beads: a study of vibrational and thermal properties using experimental and theoretical methods. J Mol Model 28, 37 (2022). https://doi.org/10.1007/s00894-022-05028-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05028-8