Abstract
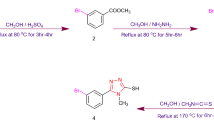
In this study, a series of arylated triazine–based sulfanilamide derivatives was designed and synthesized via palladium (Pd-0)-catalyzed, Suzuki–Miyaura cross-coupling. The theoretical investigation of the optoelectronic attributes of the synthesized compounds was accomplished using Gaussian-09 package by Density Functional Theory (DFT) approach at WB97XD/6-31G (d, p). The triazine core was stabilized by the electron-rich environment around the HOMO orbital which in turn ensured the availibity of electrons for complexation of the Pd center. The computations in DFT were carried out for better comprehension of structure–property relationship. The theoretically computed values were in good agreement with the experimental findings. The outcomes of this investigation validated importance of mono- and di-substituted arylated triazine–based sulfanilamide derivatives in organic electronics. The structural identity of newly synthesized compounds was confirmed by 1H NMR, 13C NMR, and EI-MS analyses.















Similar content being viewed by others
Data availability
All data will be available if required.
Code availability
Not applicable.
References
Machakanur SS et al (2012) Synthesis, characterization and anticancer evaluation of novel tri-arm star shaped 1, 3, 5-triazine hydrazones. J Mol Struct 1011:121–127
Vielhaber G et al (2006) Sunscreens with an absorption maximum of≥ 360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts. Photochem Photobiol Sci 5(3):275–282
Tanaka H et al (2012) Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative. Chem Commun 48(93):11392–11394
Gamez P, Reedijk J (2006) 1, 3, 5-Triazine-based synthons in supramolecular chemistry. Eur J Inorg Chem 2006(1):29–42
Afonso CA, Lourenço NM, Rosatella ADA (2006) Synthesis of 2, 4, 6-tri-substituted-1, 3, 5-triazines. Molecules 11(1):81–102
Wang C et al (2017) A sequential suzuki coupling approach to unsymmetrical aryl s-triazines from cyanuric chloride. Adv Synth Catal 359(14):2514–2519
Tan JQ, Chang JH, Deng MZ (2004) Coupling reaction of organoboronic acids with chloropyrimidines and trichlorotriazine. Chin J Chem 22(9):941–944
Cammidge AN, Crépy KV (2000) The first asymmetric Suzuki cross-coupling reaction. Chem Commun 18:1723–1724
Karimi B, Safari AA (2008) One-pot synthesis of α-aminonitriles using a highly efficient and recyclable silica-based scandium (III) interphase catalyst. J Organomet Chem 693(18):2967–2970
Kertesz M, Choi CH, Yang S (2005) Conjugated polymers and aromaticity. Chem Rev 105(10):3448–3481
Veerakumar P et al (2017) Computational studies of versatile heterogeneous palladium-catalyzed Suzuki, Heck, and Sonogashira coupling reactions. ACS Sustain Chem Eng 5(10):8475–8490
Sun Y et al (2012) Solution-processed small-molecule solar cells with 6.7% efficiency. Nature Mater 11(1):44–48
Naveed A et al (2022) Tuning the optoelectronic properties of benzodithiophene based donor materials and their photovoltaic applications. Mater Sci SemicondProcess 137:106150
Zara Z et al (2017) A comparative study of DFT calculated and experimental UV/Visible spectra for thirty carboline and carbazole based compounds. J Mol Struct 1149:282–298
Khera, N., et al. Design of charge controller for solar PV systems. in 2015 International Conference on Control, Instrumentation, Communication and Computational Technologies (ICCICCT). 2015. IEEE.
Funes-Ardoiz I, Maseras F (2018) Oxidative coupling mechanisms: current state of understanding. ACS Catal 8(2):1161–1172
Hosseinnejad T, Ebrahimpour-Malmir F, Fattahi B (2018) Computational investigations of click-derived 1, 2, 3-triazoles as keystone ligands for complexation with transition metals: a review. RSC Adv 8(22):12232–12259
Mubashar U et al (2021) Designing and theoretical study of fluorinated small molecule donor materials for organic solar cells. J Mol Model 27(7):1–13
Salim M et al (2021) Tuning the optoelectronic properties of ZOPTAN core-based derivatives by varying acceptors to increase efficiency of organic solar cell. J Mol Model 27(11):1–14
Yousaf I et al (2021) Isatin-derived non-fullerene acceptors for efficient organic solar cells. Mater Sci Semicond Process 121:105345
Farhat A et al (2021) Designing and theoretical characterization of benzodithiophene dione based donor molecules for small molecule organic solar cells. Optik 242:167098
Musawwir A et al (2021) Theoretical and computational study on electronic effect caused by electron withdrawing/electron-donating groups upon the coumarin thiourea derivatives. Comput Theor Chem 1201:113271
Sikandar R et al (2021) Tuning the optoelectronic properties of oligothienyl silane derivatives and their photovoltaic properties. J Mol Graph Model 106:107918
Khan AU et al (2021) DFT study of superhalogen (AlF4) doped boron nitride for tuning their nonlinear optical properties. Optik 231:166464
Dennington, R., et al., Semichem Inc. Shawnee Mission KS, GaussView, Version, 2009. 5.
Martin JM, Bauschlicher CW Jr, Ricca A (2001) On the integration accuracy in molecular density functional theory calculations using Gaussian basis sets. Comput Phys Commun 133(2–3):189–201
Rouf SA, Mares J, Vaara J (2015) 1H chemical shifts in paramagnetic Co (II) pyrazolylborate complexes: A first-principles study. J Chem Theory Comput 11(4):1683–1691
Wei L et al (2016) Application of Gaussian 09/GaussView 5.0 in analytical chemistry teaching. J Kunming Med Univ 37(10):134–136
Beck AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–6
Corral I et al (2006) An experimental and theoretical investigation of gas-phase reactions of Ca2+ with glycine. Chem-A Eur J 12(26):6787–6796
Irfan M et al (2017) Benchmark study of UV/Visible spectra of coumarin derivatives by computational approach. J Mol Struct 1130:603–616
Bari A et al (2017) Benchmark study of bond dissociation energy of SiX (XF, Cl, Br, N, O, H and C) bond using density functional theory (DFT). J Mol Struct 1143:8–19
Pedone A (2013) Role of solvent on charge transfer in 7-aminocoumarin dyes: new hints from TD-CAM-B3LYP and state specific PCM calculations. J Chem Theory Comput 9(9):4087–4096
Carta F et al (2011) Sulfonamides incorporating 1, 3, 5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: solution and X-ray crystallographic studies. Bioorg Med Chem 19(10):3105–3119
Hussain M et al (2010) Synthesis of aryl-substituted pyrimidines by site-selective suzuki–miyura cross-coupling reactions of 2, 4, 5, 6-tetrachloropyrimidine. Adv Synth Catal 352(9):1429–1433
Ullah I et al (2009) Synthesis of tetraaryl-p-benzoquinones by Suzuki-Miyaura cross-coupling reactions of tetrabromo-p-benzoquinone. Tetrahedron Lett 50(32):4651–4653
Hussain M et al (2015) Synthesis of arylated benzofurans by regioselective Suzuki-Miyaura Cross-coupling reactions of 2, 3-dibromobenzofurans-and 2, 3, 5-tribromobenzofurans. J Heterocycl Chem 52(2):497–505
Khera RA et al (2012) Regioselective arylation and alkynylation of 2, 3-dibromo-1H-inden-1-one by Suzuki Miyaura and Sonogashira Cross-Coupling Reactions. Helv Chim Acta 95(3):469–482
Khera RA et al (2012) Site-selective suzuki–Miyaura and Sonogashira Cross coupling reactions of the bis (triflate) of 2, 4′-bis (hydroxy) diphenyl sulfone. ChemCatChem 4(3):356
Khera RA et al (2012) Synthesis of functionalized 3, 4-diarylbenzophenones and 2, 4-diarylbenzophenones by site-selective suzuki and sonogashira cross-coupling reactions of bis (triflates) of 3, 4-and 2, 4-dihydroxybenzophenone. Synthesis 44(02):219–234
Tognetti V et al (2013) A proposal for an extended dual descriptor: a possible solution when frontier molecular orbital theory fails. Phys Chem Chem Phys 15(34):14465–14475
Zhuo LG, Liao W, Yu ZX (2012) A frontier molecular orbital theory approach to understanding the Mayr equation and to quantifying nucleophilicity and electrophilicity by using HOMO and LUMO energies. Asian J Org Chem 1(4):336–345
Fukui K, Yonezawa T, Shingu H (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20(4):722–725
Bendjeddou, A., et al., Molecular structure, HOMO-LUMO, MEP and Fukui function analysis of some TTF-donor substituted molecules using DFT (B3LYP) calculations. Int Res J Pure Appl Chem, 2016 1–9
Marahatta AB (2020) Computational study on electronic structure, atomic charges distribution and frontier molecular orbitals of butadiene: general features for diels-alder reaction. Int J Progress Sci Technol 19(2):48–64
Marahatta AB (2020) DFT study on electronic charge distribution and quantum− chemical descriptors for the kinetic stability of vanadium aquo complex ions [V (H2O) 6] 2+ and [V (H2O) 6] 3+. Int J Progress Sci Technol 22(1):67–81
Demircioğlu Z, Kaştaş ÇA, Büyükgüngör O (2015) Theoretical analysis (NBO, NPA, Mulliken Population Method) and molecular orbital studies (hardness, chemical potential, electrophilicity and Fukui function analysis) of (E)-2-((4-hydroxy-2-methylphenylimino) methyl)-3-methoxyphenol. J Mol Struct 1091:183–195
Saranya M et al (2018) Molecular structure, NBO and HOMO-LUMO analysis of quercetin on single layer graphene by density functional theory. Dig J Nanomater Biostruct 13(1):97–105
Acknowledgements
Financial support from the Higher Education Commission (HEC) of Pakistan for this project is gratefully appreciated. We are also thankful to Dr. Khurshid Ayub, COMSATS University, Islamabad, Abbottabad Campus, Pakistan for additional resources.
Funding
Funding acquisition is from the University of Agriculture Faisalabad (UAF), Pakistan.
Author information
Authors and Affiliations
Contributions
Iqra Khalid: writing an original draft, formal analysis. Rasheed Ahmad Khera: data curation and funding acquisition Shauakt Ali: formal analysis, data curation. Muhammad Shahid: data curation and formal analysis.
Corresponding author
Ethics declarations
Ethics approval
We approved all ethics.
Consent to participate
Not applicable.
Consent to publications
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associated information
Supporting information of all synthesized and optimized molecules along X, Y, and Z-axis at by Gaussian 09 package by DFT approach at WB97XD/6-31G (d, p) basis set.
Rights and permissions
About this article
Cite this article
Khalid, I., Khera, R.A., Ali, S. et al. Design, synthesis, and comparative study of optoelectronic properties of arylated triazine-based sulfanilamide derivatives through Suzuki–Miyaura cross-coupling reactions. J Mol Model 28, 44 (2022). https://doi.org/10.1007/s00894-022-05027-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05027-9