Abstract
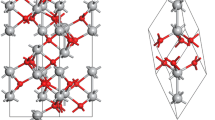
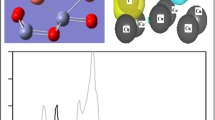
In this work, we employed continuously the DFT calculations to study CO oxidation reaction on the defective ZnO \(\left(10\overline{1 }0\right)\) surface. The oxygen (O) atom was removed from cleaned surface ZnO \(\left(10\overline{1 }0\right)\) (CS-ZnO) to form the defective ZnO \(\left(10\overline{1 }0\right)\) surface (DS-ZnO), which contained an O vacancy defect. Hereafter, the formation of oxygen vacancy was found to increase the adsorption abilities of O2 and CO on DS-ZnO, in comparison to those on CS-ZnO. Many steps of elementary reactions including O2 and CO adsorption, reacting between CO and O to form CO2, and CO2 desorption on DS-ZnO were investigated and calculated in terms of the configurations, activation energy, and reaction energy, to which the reaction pathway of CO oxidation has been found. Based on this pathway, the calculation results of the rate controlling step of 0.84 eV corresponding to the exothermic reaction energy of 4.11 eV on DS-ZnO indicated that the CO oxidation on DS-ZnO was more thermodynamically favorable and less kinetically desirable than that on CS-ZnO. In addition, the natural bonds of O2 and CO adsorptions on DS-ZnO were also analyzed by the partial density of state (PDOS) and the electron density difference (EDD) contour plots.
Graphical abstract








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Vienna ab initio Simulation Package (VASP) program.
References
Alavi A, Hu P, Deutsch T, Silvestrelli PL, Hutter J (1998) CO oxidation on Pt(111): an ab initio density functional theory study. Phys Rev Lett 80(16):3650–3653
Zhang CJ, Hu P (2001) CO oxidation on Pd(100) and Pd(111): a comparative study of reaction pathways and reactivity at low and medium coverages. J Am Chem Soc 123:1166–1172
Fajín JLC, Cordeiro MNlDS, Gomes JRB (2008) DFT study of the CO oxidation on the Au(321) surface, J. Phys. Chem. C 112, 17291–17302
Kahlich MJ, Gasteiger HA, Behm RJ (1997) Kinetics of the selective CO oxidation in H2-rich gas on Pt/Al2O3. J Catal 171:93–105
Huang M, Fabris S (2008) CO adsorption and oxidation on ceria surfaces from DFT+U calculations. J Phys Chem C 112:8643–8648
Gong XQ, Raval R, Hu P (2003) The catalytic role of water in CO oxidation. J Chem Phys 12:119
Liu Z-P, Hu P, Alavi A (2001) Mechanism for the high reactivity of CO oxidation on a ruthenium–oxide. J Chem Phys 114(13):5956–5957
Haryanto A, Fernando S, Murall N, Adhikari S (2005) Current status of hydrogen production techniques by steam reforming of ethanol: a review. Energy Fuels 19:2098–2106
Dulub O, Boatner LA, Diebold U (2002) STM study of the geometric and electronic structure of ZnO(0001)-Zn, (000–1)-O, (10–10), and (1–220) surfaces. Surf Sci 519:201–217
Hu J, Guo W-P, Shi X-R, Li B-R, Wang J (2009) Copper deposition and growth over ZnO nonpolar (101j0) and (112j0) surfaces: a density functional theory study. J Phys Chem C 113:7227–7235
Wander A, Harrison NM (2003) The structure of higher defective ZnO (10–10). Surf Sci 529:L281–L284
Meyer B, Marx D (2003) Density-functional study of the structure and stability of ZnO surfaces, Phys Rev Lett B 67, 035403
Vo CT, Huynh LK, Hung J-Y, Jiang J-C (2013) Methanol adsorption and decomposition on ZnO(1010) surface: a density functional theory study., Applied. Surface. Science. 280, 219–224
Pala WT RGS, Sushchikh MM, Park J-N, Forman AJ, Wua G, Kleiman-Shwarsctein A, Zhang J, McFarland EW, Metiu H (2009) CO oxidation by Ti- and Al-doped ZnO: oxygen activation by adsorption on the dopant, Journal of Catalysis 266, 50–58
Gracia JM, Prinsloo FF, Niemantsverdriet JW (2009) Mars-van Krevelen-like mechanism of CO hydrogenation on an iron carbide surface. Catal Lett 133:257–261
Ruiz Puigdollers A, Schlexer P, Tosoni S, Pacchioni G (2017) Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catalysis 7(10):6493–6513
Acharya CK, Turner CH (2008) CO oxidation with Pt(111) supported on pure and boron-doped carbon: a DFT investigation. Surf Sci 602:3595–3602
Camellone MF, Fabris S (2009) Reaction mechanisms for the CO oxidation on Au/CeO2 catalysts: activity of substitutional Au3+/Au+ cations and deactivation of supported Au+ adatoms. J Am Chem Soc 131:10473–10483
Jen SF, Anderson AB (1989) CO oxidation mechanisms over ZnO: Molecular orbital theory. Surf Sci 223:119–130
Meyer B, Marx D (2003) First-principles study of CO adsorption on ZnO surfaces, J. Phys.: Condens. Matter 15, L89-L94.
Lee SA, Jeong H, Woo S, Hwang J-Y, Choi S-Y, Kim S-D, Choi M, Roh S, Yu H, Hwang J, Kim SW, Choi WS (2016) Phase transitions via selective elemental vacancy engineering in complex oxide thin films. Sci Rep 6(1):23649
Hinuma TTY, Kamachi T, Maeno Z, Takakusag S, Furukawa S, Takigawa C, Shimizu K (2018) Density functional theory calculations of oxygen vacancy formation and subsequent molecular adsorption on oxide surfaces, J Phys Chem C 122, 29435−29444
Tanaka FO Isao (2002) Kazuyoshi Tatsumi, Masahiro Kunisu, Masanobu Nakano and Hirohiko Adachi, Theoretical formation energy of oxygen-vacancies in oxides. Materials Transactions 43(7):1426–1429
McCluskey MD, Jokela SJ (2009) Defect in ZnO. J. Appl. Phys. 106:071101
Walle AJaCGVd (2009) Fundamentals of zinc oxide as a semiconductor, Rep Prog Phys 72, 126501
Chen S-HL W-K, Mei-Juan Cao, Qian-Gu Yan, Chun-Hai Lu, Adsorption and dissociation of methanol on Au(1 1 1) surface: a first-principles periodic density functional study, Journal of Molecular Structure: THEOCHEM 770 (2006) 87–91.
Kuang X-J, Wang X-Q, Liu G-B (2011) A density functional study on the adsorption of hydrogen molecule onto small copper clusters. J Chem Sci 123(5):743–754
Pinto VYSFM, Silva RC, La Porta FA (2019) Oxygen defects and surface chemistry of reducible oxides. Frontiers in Material 6:260
Desireddy A, Conn BE, Guo J, Yoon B, Barnett RN, Monahan BM, Kirschbaum K, Griffith WP, Whetten RL, Landman U, Bigioni TP (2013) Ultrastable silver nanoparticles. Nature 501(7467):399–402
Rangel T, Rignanese G-M, Olevano V (2015) Can molecular projected density of states (PDOS) be systematically used in electronic conductance analysis? Beilstein J Nanotechnol 6:1247–1259
Oshikiri M, Ye J, Boero M (2014) Inhomogeneous RVO4 photocatalyst systems (R = Y, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). The Journal of Physical Chemistry C 118(16):8331–8341
Harrison JF (2003) On the role of the electron density difference in the interpretation of molecular properties. J Chem Phys 119(16):8763–8764
Davidson ER, Chakravorty S (1992) A test of the Hirshfeld definition of atomic charges and moments. Theoret Chim Acta 83(5):319–330
Szyja BM (2018) 2-Electron reduction of CO2 by graphene supported ru complexes – on the role of electron donation. Chem Electro Chem 5(15):2105–2112
Huang Y, Gao Y, Zhang Q, Zhang Y, Cao J-J, Ho W, Lee SC (2018) Biocompatible FeOOH-Carbon quantum dots nanocomposites for gaseous NOx removal under visible light: improved charge separation and High selectivity. J Hazard Mater 354:54–62
Zhang X, Li T, Guan M, Li R, Liu J, Wang Y, Zhang C, Fan C (2021) DFT insights into the migration of effective electrons towards O2 for OH formation over electron-rich sites on BiOBr (001) surface. Applied Surface Science 567:150828
Dong XA, Zhang W, Sun Y, Li J, Cen W, Cui Z, Huang H, Dong F (2018) Visible-light-induced charge transfer pathway and photocatalysis mechanism on Bi semimetal@defective BiOBr hierarchical microspheres. Journal of Catalysis 357:41–50
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mat Sci 6, 15
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys Rev B 49:14251
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47:558
Blochl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Erratum: Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 48:4978
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Olsen RA, Kroes GJ, Henkelman G, Arnaldsson A, Jónsson H (2004) Comparison of methods for finding saddle points without knowledge of the final states. J. Chem. Phys. 121(20):9776
Henkelman G, Uberuaga BP, Jónsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113(22):9901
Henkelman G, Jónsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113(22):9978
Fiorentini V, Methfessel M (1996) Extracting convergent surface energies from slab calculations. J Phys: Condens Matter 8(36):6525–6529
Van de Walle CG, Neugebauer J (2004) First-principles calculations for defects and impurities: applications to III-nitrides. J Appl Phys 95(8):3851–3879
Hine NDM, Frensch K, Foulkes WMC, Finnis MW (2009) Supercell size scaling of density functional theory formation energies of charged defects. Physical Review B 79(2):024112
Pala RG S, Metiu H (2007) Modification of the oxidative power of ZnO(101h0) surface by substituting some surface Zn atoms with other metals. J. Phys. Chem. C 111:8617–8622
Hinuma Y, Mine S, Toyao T, Kamachi T, Shimizu K-I (2021) Factors determining surface oxygen vacancy formation energy in ternary spinel structure oxides with zinc. Phys Chem Chem Phys 23(41):23768–23777
H.M. Raj Ganesh S. Pala, Selective promotion of different modes of methanol adsorption via the cation substitutional doping of a ZnO(10¯10) surface, Journal of Catalysis 254 (2008) 325–331.
Acknowledgements
We are also grateful to the National Center of High-Performance Computing (NCHC) for donating computer time and facilities.
Funding
This work was financed by the Industrial University of Ho Chi Minh City in the project with the core of contractual 21/1H06.
Author information
Authors and Affiliations
Contributions
Dr. Vo Thanh Cong: supervision, conceptualization, methodology, software, data analyses, writing the original draft, review and editing. Dr. Nguyen Van Son: review and editing. Dr. Son Quynh Thai Pham: supervision, writing, data analyses, review and editing.
Corresponding authors
Ethics declarations
Ethics approval
Our manuscript has not been submitted to more than one journal for simultaneous consideration.
Our manuscript has not been published previously (partly or in full).
A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (e.g., “salami-publishing”).
No data have been fabricated or manipulated (including images) to support our conclusions.
No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”).
Consent to participate
Our current study did not involve human subject, and has not used living animals, etc.
Consent for publication
The authors agree to publish this work on the Journal of Molecular Modeling.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to addition of ORCID to author Son Quynh Thai Pham.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cong, V.T., Van Son, N. & Pham, S.Q.T. A comparison of CO oxidation on cleaned ZnO \((10\overline{1 }0)\) surface and defective ZnO \((10\overline{1 }0)\) surface using density functional theory studies. J Mol Model 28, 12 (2022). https://doi.org/10.1007/s00894-021-05011-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-05011-9