Abstract
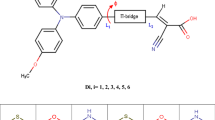
The investigation of dye-sensitized solar cells (DSSCs) based on different donor groups linked with cyanoacrylic acid electron acceptor by Selenophene as π-bridged (D-π-A) was performed based on density functional theory (DFT) time-dependent DFT (TDDFT). Different functional were tested W97XD, PBEPBE, CAM-B3LYP, and B3PW91, and compared with experimental results of the reference D1. The theoretical results with CAM-B3LYP functional at 6-311G (d,p) basis sets were capable of predicting the absorption maximum that has been reported experimentally. Calculations were made to establish the conformational orientation of the cyanoacrylic acid group and evaluate the effect of changing donor units' on the electronic properties of the ground state. Structural and electronic properties, along with the photovoltaic properties, were investigated. The LUMO and HOMO energy levels of these dyes can positively affect the process of electron injection and dye regeneration. Light-harvesting efficiency (LHE), injection driving force (ΔGinject), and total reorganization energy (total) were also discussed. To further support the previous proprieties, electronic excited state energies were obtained by TDDFT// CAM-B3LYP/6-311G(d,p) calculations. The calculated results of these dyes reveal that D8 dye possessing triphenylamine donor unit has the best electronic, optical properties, and photovoltaic parameters.






Similar content being viewed by others
Data availability
Not applicable
References
Ananthakumar S, Ramkumar J, Babu SM (2014) Effect of co-sensitization of cdse nanoparticles with n3 dye on tio2 nanotubes. Sol Energy 106:136–142
Asbury JB, Wang Y-Q, Hao E, Ghosh HN, Lian T (2001) Evidences of hot excited state electron injection from sensitizer molecules to tio 2 nanocrystalline thin films. Res Chem Intermed 27(4):393–406
Aslan E, Gonce MK, Yigit MZ, Sarilmaz A, Stathatos E, Ozel F, Can M, Patir IH (2017) Photocatalytic h2 evolution with a cu2ws4 catalyst on a metal free d-n-a organic dye-sensitized tio2. Appl Catal B Environ 210:320–327
Balanay MP, Kim DH (2008) Dft/td-dft molecular design of porphyrin analogues for use in dye-sensitized solar cells. Phys Chem Chem Phys 10(33):5121–5127
Bisquert J, Cahen D, Hodes G, Ruhle S, Zaban A (2004) Physical chemical principles of photovoltaic conversion with nanoparticulate, mesoporous dye-sensitized solar cells. J Phys Chem B 108(24):8106–8118
Dhar A, Kumar NS, Ibrahim AA, Vekariya RL (2018) Effective photo-harvesting by dye sensitized solar cell based on dihydrothieno [3, 4-b][1, 4] dioxine bridge based metal free organic dye. Org Electron 56:232–239
Ding W-L, Wang D-M, Geng Z-Y, Zhao X-L, Xu W-B (2013) Density functional theory characterization and verification of high-performance indoline dyes with d-a- n-a architecture for dye-sensitized solar cells. Dyes Pigments 98(1):125–135
Elsanousi A, Elamin N, Elhouri S, Abdallah A (2013) Highly ordered tio2 nanotubes and their application to dye sensitized solar cells. J Appl Industrial Sci 1(1):39–42
Fernandes SS, Castro MCR, Mesquita I, Andrade L, Mendes A, Raposo MMM (2017) Synthesis and characterization of novel thieno [3, 2-b] thiophene based metal- free organic dyes with different heteroaromatic donor moieties as sensitizers for dye- sensitized solar cells. Dyes Pigments 136:46–53
Guillaumont D, Nakamura S (2000) Calculation of the absorption wavelength of dyes using time-dependent density-functional theory (td-dft). Dyes Pigments 46(2):85–92
Hagfeldt A, Gratzel M (2000) Molecular photovoltaics. Acc Chem Res 33(5):269–277
Hardin BE, Snaith HJ, McGehee MD (2012) The renaissance of dye-sensitized solar cells. Nat Photonics 6(3):162–169
Jacquemin D, Mennucci B, Adamo C (2011) Excited-state calculations with td-dft: from benchmarks to simulations in complex environments. Phys Chem Chem Phys 13(38):16987–16998
Jung HS, Lee J-K (2013) Dye sensitized solar cells for economically viable photovoltaic systems. J Phys Chem Lett 4(10):1682–1693
Kakiage K, Aoyama Y, Yano T, Oya K, Kyomen T, Hanaya M (2015) Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem Commun 51(29):6315–6317
Kamat PV, Haria M, Hotchandani S (2004) C60 cluster as an electron shuttle in a ru (ii)-polypyridyl sensitizer-based photochemical solar cell. J Phys Chem B 108(17):5166–5170
Katoh R, Furube A, Yoshihara T, Hara K, Fujihashi G, Takano S, Murata S, Arakawa H, Tachiya M (2004) Efficiencies of electron injection from excited n3 dye into nanocrystalline semiconductor (zro2, tio2, zno, nb2o5, sno2, in2o3) films. J Phys Chem B 108(15):4818–4822
Le Bahers T, Bremond E, Ciofini I, Adamo C (2014) The nature of vertical excited states of dyes containing metals for dssc applications: insights from td-dft and density based indexes. Phys Chem Chem Phys 16(28):14435–14444
Lee JH, Park GE, Choi S, Lee DH, Um HA, Shin J, Cho MJ, Choi DH (2016) Effect of the thiophene and selenophene moiety in regular terpolymers on the performance of thin film transistors and polymer solar cells. Polymer 94:43–52
Liao X, Zhang H, Huang J, Wu G, Yin X, Hong Y (2018) (d- n- a) 3- type metal-free organic dye for dye-sensitized solar cells application. Dyes Pigments 158:240–248
Liu L, Ren Z, Xiao C, Dong H, Yan S, Hu W, Wang Z (2016) Surface-induced highly oriented perylo [1, 12-b, c, d] selenophene thin films for high performance organic field- effect transistors. Org Electron 35:186–192
Luo J, Wan Z, Jia C, Wang Y, Wu X, Yao X (2016) Co-sensitization of dithiafulvenyl- phenothiazine based organic dyes with n719 for efficient dye-sensitized solar cells. Elec- trochimica Acta 211:364–374
Narayan MR (2012) Dye sensitized solar cells based on natural photosensitizers. Renew Sust Energ Rev 16(1):208–215
Nosheen E, Shah SM, Hussain H, Murtaza G (2016) Photo-sensitization of zns nanoparticles with renowned ruthenium dyes n3, n719 and z907 for application in solid state dye sensitized solar cells: A comparative study. J Photochem Photobiol B Biol 162:583–591
Novir SB, Hashemianzadeh SM (2014) Computational investigation of low band gap dyes based on 2-styryl-5-phenylazo-pyrrole for dye-sensitized solar cells. Curr Appl Phys 14(10):1401–1410
Sajjad S, Hashmi MA, Mahmood T, Ayub K (2019) Density functional theory study of structural, electronic and co adsorption properties of anionic scn-(n= 2-13) clusters. Comput Theoret Chem 1163:112511
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of monascus dyes as photosensitizer for dye-sensitized solar cells. J Photochem Photobiol A Chem 236:35–40
Sharmoukh W, Hassan ZM, Ali BA, Elnagar MM, Abdo RM, Allam NK (2018) Position of the anchoring group determined the sensitization efficiency of metal-free d-n-a dyes: Combined experimental and td-dft insights. J Photochem Photobiol A Chem 367:128–136
Shoute LC, Loppnow GR (2002) Excited-state dynamics of alizarin-sensitized tio 2 nanoparticles from resonance raman spectroscopy. J Chem Phys 117(2):842–850
Sun C, Li Y, Song P, Ma F (2016) An experimental and theoretical investigation of the electronic structures and photoelectrical properties of ethyl red and carminic acid for dssc application. Materials 9(10):813
Tamba S, Fujii R, Mori A, Hara K, Koumura N (2011) Synthesis and properties of seleno-analog mk-organic dye for photovoltaic cells prepared by c-h functionalization reactions of selenophene derivatives. Chem Lett 40(9):922–924
Tamilavan V, Park JB, Kang I-N, Hwang D-H, Hyun MH (2014) Benzodithiophene-based polymers containing novel electron accepting selenophene- incorporated pyrrolo [3, 4-c] pyrrole-1, 3-dione units for highly efficient thin film transistors and polymer solar cells. Synth Met 198:230–238
Tang A, Xiao B, Wang Y, Gao F, Tajima K, Bin H, Zhang Z-G, Li Y, Wei Z, Zhou E (2018) Simultaneously achieved high open-circuit voltage and efficient charge generation by fine-tuning charge-transfer driving force in nonfullerene polymer solar cells. Adv Funct Mater 28(6):1704507
Wang X, Huang J, Tajima K, Xiao B, Zhou E (2015) An amorphous n-type polymer based on perylenediimide and selenophene for all-polymer solar cells application. Mat Today Communicat 4:16–21
Zhang C-R, Liu Z-J, Chen Y-H, Chen H-S, Wu Y-Z, Feng W, Wang D-B (2010) Dft and td-dft study on structure and properties of organic dye sensitizer ta-st-ca. Curr Appl Phys 10(1):77–83
Zhang J, Zhu H-C, Zhong R-L, Wang L, Su Z-M (2018) Promising heterocyclic anchoring groups with superior adsorption stability and improved ipce for high-efficiency noncarboxyl dye sensitized solar cells: A theoretical study. Org Electron 54:104113
Zhang T-T, Jia J, Wu H-S (2012) Theoretical studies of cooh group effect on the performance of rhenium (i) tricarbonyl complexes with bispyridine sulfur-rich core ligand as dyes in dssc. Theor Chem Accounts 131(9):1–8
Zhang X, Wang Q, Liang Z, Li M, Geng Y (2019) Low-bandgap non-fullerene acceptors based on selenophene n spacer and alkylated indaceno dithiophene for organic solar cells. Org Electron 69:200–207
Code availability
Not applicable
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouaamlat, H., Abram, T., Bouachrine, M. et al. Organic dyes based on selenophene for efficient dye-sensitized solar cell. J Mol Model 27, 333 (2021). https://doi.org/10.1007/s00894-021-04953-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04953-4