Abstract
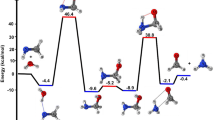
The O(3P)-initiated conversion mechanism and dynamics of CH3CHCO were researched in atmosphere by executing density functional theory (DFT) computations. Optimizations of all the species and single-point energy computations were implemented at the B3LYP/6–311++G(d,p) and CCSD(T)/cc-pVTZ level, respectively. The explicit oxidation mechanism was introduced and discussed. The results state clearly that the O(3P) association was more energetically beneficial than the abstraction of H. The rate coefficients over the probable temperature range of 200–3000 K were forecasted by implementing Rice-Ramsperger-Kassel-Marcus (RRKM) theory. Specifically, the total rate coefficient of O(3P) association reactions is 1.19 × 10−11 cm3 molecule−1 s−1 at 298 K, which is consistent with the experimental results (1.16 × 10−11 cm3 molecule−1 s−1). The rate coefficients for the O(3P) with CH2CO, CH3CHCO, and (CH3)2CCO suggest that rate coefficient of ketene derivatives increase with the increase of methylation degree.

Graphical abstract








Similar content being viewed by others

References
Frank P, Bhaskaran KA, Just T (1986) High-temperature reactions of triplet methylene and ketene with radicals. J. Phys. Chem. 90:3809–3814
Sun H, Tang YZ, Wang ZL, Pan XM, Li ZS, Wang RS (2005) DFT study on the mechanisms of the CH2CO + NCX (X = O, S) reactions. J. Mol. Struct. THEOCHEM 757:143–148
Washida N, Hatakeyama S, Takagi H, Kyogoku T, Sato S (1983) Reaction of ketenes with atomic oxygen. J. Chem. Phys. 78:4533–4540
Gaffney JS, Atkinson R, Pitts Jr JN (1975) Relative rate constants for the reaction of O(3P) atoms with selected olefins, monoterpenes, and unsaturated aldehydes. J. Am. Chem. Soc. 97:6481–6483
Mack GPR, Thrush BA (1974) Reaction of oxygen atoms with carbonyl compounds part 3. – ketene. J. Chem. Soc. Faraday Trans. 1(70):187–192
Zhou ZY, Fu H, Zhou XM, Cheng XL (2003) Mechanistic investigation on the multi-channel reaction of Cl+CH2CO. J. Mol. Struct. THEOCHEM 620:207–214
Wallington TJ, Ball JC, Straccia AM, Hurley MD, Kaiser EW, Dill M, Schneider WF (1996) Kinetics and mechanism of the reaction of Cl atoms with CH2CO (ketene). Int. J. Chem. Kinet. 28:627–635
Maricq MM, Ball JC, Straccia AM, Szente JJ (1997) A diode laser study of the Cl + CH3CO reaction. Int. J. Chem. Kinet. 29:421–429
Hou H, Wang BS, Gu Y (2000) Ab initio mechanism and multichannel RRKM-TST rate constant for the reaction of Cl(2P) with CH2CO (ketene). J. Phys. Chem. A 104:320–328
Grussdorf J, Nolte J, Temps F, Wagner HGG (1994) Primary products of the elementary reactions of CH2CO with F, Cl, and OH in the gas phase Ber. Bunsenges. Phys. Chem. 98:546–553
Cheng XL, Zhao YY, Zhou XM, Zhou ZY (2003) Reaction mechanism for the F + CH2COreaction system based on density functional theory and vibrational mode analysis. J. Mol. Struct. THEOCHEM 638:27–35
Lee J, Bozzelli JW (2003) Reaction of H plus ketene to formyl methyl and acetyl radicals and reverse dissociations. Int. J. Chem. Kinet. 35:20–44
Michael JV, Nava DF, Payne WA, Stief LJ (1979) Absolute rate constants for the reaction of atomic hydrogen with ketene from 298 to 500 K. J. Chem. Phys. 70:5222–5227
Slemr F, Warneck P (1975) Reactions of atomic hydrogen with ketene and acetaldehyde. Ber. Bunsenges. Phys. Chem. 79:152–156
Hatakeyama S, Honda S, Akimoto H (1985) Reactions of ketene, methylketene, ethylketene, and dimethylketene with ozone in air. Bull. Chem. Soc. Jpn. 58:2411–2412
Oehlers C, Temps F, Wagner HGG, Wolf M (1992) Kinetics of the reaction of OH radicals with CH2CO. Ber. Bunsenges. Phys. Chem. 96:171–175
Brown AC, Canosa-Mas CE, Parr AD, Wayne RP (1989) Temperature dependence of the rate of the reaction between the OH radical and ketene. Chem. Phys. Lett. 161:491–496
Semenikhin AS, Shubina EG, Savchenkova AS, Chechet IV, Matveev SG, Konnov AA, Mebel AM (2018) Mechanism and rate constants of the CH3 + CH2CO reaction: a theoretical study. Int. J. Chem. Kinet. 50:273–284
Sun H, He HQ, Hong B, Chang YF, An Z, Wang RS (2006) Theoretical study of the mechanism of CH2CO + CN reaction. Int. J. Quant. Chem. 106:894–905
Kohn W, Sham LJ (1965a) Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. A 137:1697–1705
Sham LJ (1965b) Self-consistent equations including exchange and correlation effects. Phys. Rev. A 140:1133–1138
Holbrook KA, Pilling MJ, Robertson SH (1996) Unimolecular reactions; J. Wiley, Chichester
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc, Wallingford CT
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlationenergy formula into a functional of the electron density. Phys. Rev. B 37:785–789
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98:5648–5652
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72:650–654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comp. Chem. 14:294–301
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J. Chem. Phys. 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 94:5523
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 157:479–483
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90:1007
Code availability
Gaussian 09 package program.
Funding
This work was supported by the Natural Science Foundations of China (No. 21707062) and Scientific Research Starting Foundation of Mianyang Normal University (No. QD2016A007) and supported by the Open Project Program of Beijing Key Laboratory of Flavor Chemistry, Beijing Technology and Business University (BTBU), Beijing 100048, China.
Author information
Authors and Affiliations
Contributions
Yongguo Liu, Huaming Du, Meilian Zhao, Yuxi Sun, Huirong Li Zhiguo Wang: Calculation, data curation, formal analysis, and investigation. Yunju Zhang: calculation and writing-review and editing
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, Y., Zhao, M. et al. Theoretical investigations on mechanisms and kinetics of methylketene with O(3P) reaction in the atmosphere. J Mol Model 27, 228 (2021). https://doi.org/10.1007/s00894-021-04850-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04850-w