Abstract
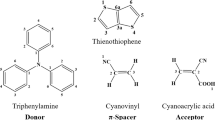
In this article, we studied a series of dye-sensitized solar cells (DSSCs) type Donor-π-Acceptor involving carbazole as donors and cyanoacrylic acid as acceptors of the electrons. These cells are linked by different π-spacer unit’s, with the aim to develop new organic dyes with high-performance optoelectronic properties. Different units have been introduced in the π-bridge in order to investigate their effects on the structural and optoelectronic properties of the studied compounds, as well as their adsorbed compounds-titanium dioxide (TiO2) semi-conductor. We evaluated and assessed the important relevant parameters that influence the performance of photovoltaic cell to measure their involvement in the short-circuit photocurrent density (Jsc). Using Density Functional Theory (DFT) and Time-Dependent-BHandHLYP, the geometrical and optoelectronics properties have been predicted theoretically. The results obtained indicate that introducing the oxazole (S5) and thiazole (S6) molecules in the π-spacer have significant impact on the geometric properties for D5-D6 dyes. This results in the fact that dye D5 has a planar structure. Also, the insertion of the thiophene, oxazole and thiazole units improves the energies of the HOMO and LUMO molecular orbitals of D1, D5, and D6 dyes. Moreover, these results show the ability of electron transfer and regeneration from the studied sensitizers (D1-D6). Also, it can be noted that the application of the pyrrole group in the π-spacer moiety of the dye (D2) improves the electron’s transfer performance with a lower regeneration motive force ΔGreg, a more negative injection driving forces (ΔGinject), and a higher values of open circuit-voltage (Voc).




Similar content being viewed by others
Availability of data and material
All data are available and mainly the Cartesian coordinates of all the studied dyes.
References
Gong J, Liang J, Sumathy K (2012) Review on dye-sensitized solar cells (DSSCs): fundamental concepts and novel materials. Renewable Susta able Energy Rev 16:5848–5860. https://doi.org/10.1016/j.rser.2012.04.044
Grätzel M (2003) Dye-sensitized solar cells. Journal of photochemistry and photobiology C. Photochem Rev 4: 145-153. https://doi.org/10.1016/S13895567(03)00026-1
Lazrak M, Toufik H, Bouzzine SM, Bih H, La chouri F (2018) Effect of π-spacers on the opto lectronic properties of triphenylamine-based organic dyes: theoretical study. RHAZES: Green Appl Chem 2:2605–6895
Ennehary S, Toufik H, Bouzzine SM, Lamchouri F (2020) Effect of the alkyl chain length on the optoelectronic properties of organic dyes: theoretical approach. J Comput Electron 19:840. https://doi.org/10.1007/s10825-020-01486-6
O'regan B, Grätzel M (1991) A low-cost, high efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nat 353:737–740
Katoh R, Furube A, Yoshihara T, Hara K, Fujihas-hi G, Takano S, Murata S, Arakawa H, Tachiya M (2004) Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2 O5, SnO2, In2 O3) films. J Phys Chem 108:4818–4822. https://doi.org/10.1021/jp031260g
Nazeeruddin MK, Kay A, Rodicio I, Humpbry Baker R, Miller E, Liska P, Vlachopoulos N, Gratzel M (1993) Conversion of light to electricity by cis-XzBis(2,2′-bipyridyl-4,4’dicarboxy-late) ruthenium( 11) charge-transfer sensitizers (X = C1-, Br-, I-, CN-, and SCN-) on nanocrystalline Ti02 electrodes. J Am Chem Soc 115(6382):6390
Hofmann M, Schellnhuber HJ (2010) Ocean acidification: a millennial challenge. Energy Eviron Sci 3:1883–1896. https://doi.org/10.1039/c000820f
Zhang CR, Liu ZJ, Chen YH, Chen HS, Wua YZ, Feng WJ, Wang DB (2010) DFT and TD-DFT study on structure and properties of organic dye sensitizer TA-St-CA. Curr Appl Phys 10:77–83. https://doi.org/10.1016/j.cap.2009.04.018
Liyanage NP, Yella A, Nazeeruddin M, Grätzel M, Delcamp JH (2016) Thieno[3,4- b ]pyrazine as an electron deficient π-bridge in D–A−π–a DSCs. ACS Appl Mater Interfaces 8:5376–5384. https://doi.org/10.1021/acsami.5b12503
Lakshmanakumar M, Sriram S, Balamurugan D (2018) Performance analysis of TiO2-flavylium compound-based dye-sensitized solar cell (DSSC ): a DFT–TDDFT approach. J Comput Electron.1 17: 1143-1152. https://doi.org/10.1007/s10825-018-1189-6
Mestiri T, Chemek M, Rouabeh J, Alimi K (2015) DFT and TD-DFT modeling of new carbazole-based oligomers for optoelectronic devices. Comput Condensed Matter 4:23–31. https://doi.org/10.1016/j.cocom.2015.07.001
Eom YK, Hong JY, Kim J, Kim HK (2017) Triphenylamine-based organic sensitizers with π- spacer structural engineering for dye-sensitized solar cells: synthesis, theoretical calculations, molecular spectroscopy and structure-property-performance relationships. Dyes Pigments 136:496–504. https://doi.org/10.1016/j.dyepig.2016.09.007
Daeneke T, Mozer AJ, Uemura Y, Makuta S, Fekete M, Tachibana Y, Koumura N, Bach U, Spiccia L (2012) Dye regeneration kinetics in dye-sensitized solar cells. J Am Chem Sco 134:16925–16928. https://doi.org/10.1021/ja3054578
Gaussian09, R.A.: 1, Frisch, mj., trucks gw., schlegelhb., scuseria, ge., robb, ma., cheeseman, jr.,Scalmani,g., Barone,v., Mennucci, b., peterson,ga. and al (2009) Gaussian Inc Wallingford CT 121: 150–166
Lazrak M, Toufik H, Bouzzine SM, Bih H, Lamchouri F (2018) The computational study of bridge effect in D-π-A photosensitive dyes, based on triphenylamine. IOP conference series. Earth Environ Sci 161:012021. https://doi.org/10.1088/1755-131 5/161/1/012021
Toufik H, Bouzzine SM, Lamchouri F, Nawdali M, Hamidi M, Bouachrine M (2012) Density functional theory study of new materials based on thiophene and oxathiazole in their neutral and doped states. J Mater Environ 3(28):6–293
Walton IM, Cox JM, Benson CA, Patel DG, Chen YS, Benedict JB (2016) The role of atropisome-rs on the p-hoto-reactivity and fatigue ofdiarylethene-based metal–organic frameworks. New J Chem 40:101–106. https://doi.org/10.1039/C5NJ01718A
Lazrak M, Toufik H, Bouzzine SM, Lamchouri F (2020) Bridge effect on the charge transfer and optoelectronic properties of triphenylamine-based organic dye sensitized solar cells: theoretical approach. Res Chem Intermed 46:3961–3978. https://doi.org/10.1007/s11164-020-04184-x
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98(1372–13):77. https://doi.org/10.1063/1.464304
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Jia C, Wan Z, Zhang J, Li Z, Yao X, Shi Y (2012) Theoretical study of carbazole–triphenyl-amine-based dyes for dye-sensitized solar cells. Spectrochim Acta A: Mol Biomol Spectroscopy 86-387-391. https://doi.org/10.1016/j.saa.2011.10.053
Figueira CA, Lopes PS, Gomes CSB, Veiros LF, Gomes PT (2015) Exploring the influence of steric hindrance and electronic nature of substituents in the supramolecular arrangements of 5-(substituted phenyl)-2-formyl pyrroles. Cryst E-ng Comm 17:6406–6419. https://doi.org/10.1039/C5CE00927H
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photo sensitizer for dye-sensitized solar cells. J Photochem Photobiol A Chem 236:35–40. https://doi.org/10.1016/j.jphotochem.2012.03.014
Cahen D, Hodes G, Grätzel M, Guillemoles JF, Riess I (2000) Nature of photovoltaic action in dye-sensitized solar cells. J Phys Chem B 104:2053–2059. https://doi.org/10.1021/jp993187t
Breitung EM, Shu CF, McMahon RJ (2000) Thiazole and thiophene analogues of donor-acceptor stilbenes: molecular hyperpolariza-bilities and structure−property relationships. J Am Chem Soc 122:1154–1160. https://doi.org/10.1021/ja-9930364
Li Y, Liu J, Liu D, Li X, Xu Y (2019) D-A-π-A based organic dyes for efficient DSSCs: a theoretical study on the role of π-spacer. Comput Mater Sci 161:163–176
Mahmood A, Khan S U D, Rana U A (2014) Theoretical designing of novel heterocyclic azodyes for dye sensitized solar cells. J Comput Ele tron 13: 1033-1041. https://doi.org/10.1007/s10825-014 0628-2
Li P, Cui Y, Song C, Zhang H (2017) A systematic study of phenoxazine-based organic sensitizers for solar cells. Dyes Pigments 137:12–23. https://doi.org/10.1016/j.dyepig.2016.09.060
Arunkumar A, Shanavas S, Anbarasan PM (2018) First-principles study of efficient phen thiazine-based D–π–A organic sensitizers with various spacers for DSSCs. J Comput Electron 17:1410–1420. https://doi.org/10.1007/s10825-018-1226-5
Namuangruk S, Fukuda R, Ehara M, Meeprasert J, Khanasa T, Morada S, Kaewin T, Jungsuttiwong S, Sudyoads-uk T, Promarak V (2012) D–D−π–A-type organic dyes for dye-sensitized solar cells with a potential for direct electron injection and a high extinction coefficient: synthesis, characterization, and theoretical investigation. J Phys Chem 116:25653–25663. https://doi.org/10.1021/jp304489t
Fahim ZME, Bouzzine SM, Youssef AA, Bouachrine M, Hamidi M (2018) Ground state geometries, UV/Vis absorption spectra and charge transfer properties of triphenylamine-thiophenes based dyes for DSSCs: a TD-DFT benchmark study. Comput Theor Chem 1125:39–48. https://doi.org/10.1016/j.comptc.2018.01.002
Zhang Q, Guo X, Huang X, Huang S, Li D, Luo Y, Shen Q, Toyoda T, Meng Q (2011) Highly efficient Cds/C-dSe-sensitized solar cells controlled by the structural properties of compact porous TiO2 photoelectrodes. Phys Chem ChemPhys 13:4659–4667. https://doi.org/10.1039/c0cp02099k
Hamann TW, Jensen RA, Martinson ABF, Rys-wyk HV, Hupp JT (2008) Advancing beyond current generation dye-sensitized solar cells. Energy Environ Sci 1:66–78
Li X, Zhang X, Hua J, Tian H (2017) Molecular engineering of organic sensitizers with o,p-dialkoxyphenyl-based bulky donors for highly efficient dye-sensitized solar cells. Mol Syst Des Eng 2:98–122. https://doi.org/10.1039/C7ME00002B
Jihuai W, Zhang L, Jianming L, Miaoliang H, Yunfang H, Leqing F, Genggeng L (2015) Electrolytes in dye-sensitized solar cells. Chem Rev 115:2136–2173. https://doi.org/10.1021/cr400675m
Grätzel M (2009) Recent advances in sensitized mesoscopic solar cells. Acc Chem Res 42:1788–1798. https://doi.org/10.1021/ar900141y
Lin BC, Cheng CP, Lao ZPM (2003) Reorganization energies in the transports of holes and electrons in organic amines in organic electroluminescence studied by density functional theory. J Phys Chem A 107:5241–5251. https://doi.org/10.1021/jp0304529
Tavernier HL, Fayer MD (2001) Solute–solute spatial distribution in hydrogen bonding liquids probed with time-dependent intermolecular electron transfer. J Chem Phys 114:4552–4564. https://doi.org/10.1063/1.1349705
Bromley ST, Mas-Torrent M, Hadley P, Rovira C (2004) Importance of intermolecular interactions in assessing hopping mobilities in organic field effect transistors: pentacene versus dithiophene-tetrathiafulvalene. J Am Chem Soc 126:6544–6545. https://doi.org/10.1021/ja049762a
Hutchison GR, Ratner MA, Marks T (2005) Hopping transport in conductive heterocyclic oligomers: reorganization energies and substituent effects. J Am Chem Soc 127:2339–2350. https://doi.org/10.1021/ja0461421
Zhang Z L, Zou L Y, Ren A. M, Liu Y F, Feng JK., Sun C C (2013) Theoretical studies on the electronic structures and optical properties of star-shaped triazatruxene/heterofluorene co-polymers. Dyes Pigments 96: 349–363. https://doi.org/10.1016/j.dyepig.2012.08.020
Estrella LL, Balanay MP, Kim DH (2016) The effect of donor group rigidification on the electronic and optical properties of arylamine-based metal-free dyes for dye-sensitized solar cells: a computational study. J Phys Chem A 120:5917–5927. https://doi.org/10.1021/acs.jpca.6b0327
Curioni A, Boero M, Andreoni W (1998) Alq3: ab initio calculations of its structural and electronic properties in neutral and charged states. Chem Phys Lett 294:263–271. https://doi.org/10.1016/S0009-2614(98)00829-X
Zou LY, Ren AM, Feng JK, Liu YL, Ran XQ, Sun CC (2008) Theoretical study on photophysical properties of multifunctional electroluminescent molecules with different π-conjugated bridges. J Phys Chem A 112:12172–12178. https://doi.org/10.1021/jp8032462
Asbury JB, Wang YQ, Hao E, Ghosh HN, Lian T (2001) Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res Chem Intermed 27:393–406. https://doi.org/10.1163/156856701104202255
Huang SY, Schlichthörl G, Nozik AJ, Grätzel M, Frank AJ (1997) Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J Phys Chem B 101:2576–2582. https://doi.org/10.1021/jp962377q
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photo sensitizer for dye-sensitized solar cells. J Photo-chem Photobiol Chem 236:35–40. https://doi.org/10.1016/j.jphotochem.2012.03.014
Author information
Authors and Affiliations
Contributions
SE did most of the practical work as part of a PhD thesis supervised by HT and prepared the manuscript. HT designed and coordinated the study, participated in article preparation, corrected the manuscript and edited the final version and submitted it for publication. ML contributed to data analysis. FL and SMB participated in study designed, helped to improve the manuscript and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Compliance with ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ennehary, S., Toufik, H., Lazrak, M. et al. Computational study of the effect of π-spacers on the optoelectronic properties of carbazole-based organic dyes. J Mol Model 27, 122 (2021). https://doi.org/10.1007/s00894-021-04733-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04733-0