Abstract
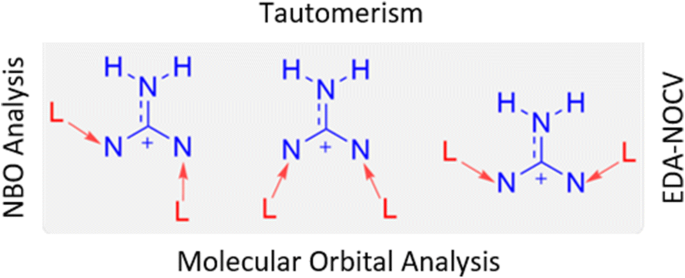
Guanidinium species are highly basic and hence mostly exist in cationic state. Because these cations carry electron-deficient centers, they can be stabilized with the help of electron-donating ligands like N-heterocyclic carbenes. A few novel guanidinium cationic species stabilized by electron-donating ligands were designed and quantum chemically evaluated. It was shown that strong hydrogen bonds and tautomerism are the important characteristics of these species. Further, the possibility of donor→acceptor coordination interactions in these species have been explored between the electron-donating carbenes and the central guanidinium unit. The results suggest that the title compounds can be considered as ligand-stabilized guanidinium cations similar to the ligand-stabilized N+ and N3+ centers.
Graphical abstract











Similar content being viewed by others
Data availability
Electronic Supporting Information ESI uploaded.
References
Vazdar K, Kunetskiy R, Saame J, Kaupmees K, Leito I, Jahn U (2014) Very strong organosuperbases formed by combining imidazole and guanidine bases: synthesis, structure, and basicity. Angew Chem Int Ed 53(5):1435–1438
Štrukil V, Lekšić E, Meštrović E, Eckert-Maksić M (2014) The synthesis and structural characterization of peralkylated triguanide superbases. Aust J Chem 67(7):1129–1133
Loh YK, Ángeles Fuentes M, Vasko P, Aldridge S (2018) Successive protonation of an N-heterocyclic imine derived carbonyl: superelectrophilic dication versus masked acylium ion. Angew Chem 130(50):16797–16801
Blondeau P, Segura M, Pérez-Fernández R, de Mendoza J (2007) Molecular recognition of oxoanions based on guanidinium receptors. Chem Soc Rev 36(2):198–210
Schug KA, Lindner W (2005) Noncovalent binding between guanidinium and anionic groups: focus on biological-and synthetic-based arginine/guanidinium interactions with phosph [on] ate and sulf [on] ate residues. Chem Rev 105(1):67–114
Best MD, Tobey SL, Anslyn EV (2003) Abiotic guanidinium containing receptors for anionic species. Coord Chem Rev 240(1–2):3–15
Hargrove AE, Nieto S, Zhang T, Sessler JL, Anslyn EV (2011) Artificial receptors for the recognition of phosphorylated molecules. Chem Rev 111(11):6603–6782
Young T, Abel R, Kim B, Berne BJ, Friesner RA (2007) Motifs for molecular recognition exploiting hydrophobic enclosure in protein–ligand binding. Proc Natl Acad Sci 104(3):808–813
Kataev EA, Müller C, Kolesnikov GV, Khrustalev VN (2014) Guanidinium-based artificial receptors for binding orthophosphate in aqueous solution. Eur J Org Chem 2014(13):2747–2753
Rozas I, Kruger PE (2005) Theoretical study of the interaction between the guanidinium cation and chloride and sulfate anions. J Chem Theory Comput 1(5):1055–1062
Campbell RK, White Jr JR, Saulie BA (1996) Metformin: a new oral biguanide. Clin Ther 18(3):360–371
Kathuria D, Bankar AA, Bharatam PV (2018) “What’s in a structure?” The story of biguanides. J Mol Struct 1152:61–78
De Greef TF, Smulders MM, Wolffs M, Schenning AP, Sijbesma RP, Meijer E (2009) Supramolecular polymerization. Chem Rev 109(11):5687–5754
Fathalla M, Lawrence CM, Zhang N, Sessler JL, Jayawickramarajah J (2009) Base-pairing mediated non-covalent polymers. Chem Soc Rev 38(6):1608–1620
de Greef TF, Meijer E (2008) Materials science: supramolecular polymers. Nature 453(7192):171
Blight BA, Hunter CA, Leigh DA, McNab H, Thomson PI (2011) An AAAA–DDDD quadruple hydrogen-bond array. Nat Chem 3(3):244–248
Seipp CA, Williams NJ, Bryantsev VS, Custelcean R, Moyer BA (2015) A conformationally persistent pseudo-bicyclic guanidinium for anion coordination as stabilized by dual intramolecular hydrogen bonds. RSC Adv 5(130):107266–107269
Zhao L, Pan S, Holzmann N, Schwerdtfeger P, Frenking G (2019) Chemical bonding and bonding models of main-group compounds. Chem Rev 119(14):8781–8845
Doddi A, Peters M, Tamm M (2019) N-Heterocyclic carbene adducts of main group elements and their use as ligands in transition metal chemistry. Chem Rev 119(12):6994–7112
Nesterov V, Reiter D, Bag P, Frisch P, Holzner R, Porzelt A, Inoue S (2018) NHCs in main group chemistry. Chem Rev 118(19):9678–9842
Patel N, Sood R, Bharatam PV (2018) NL2+ systems as new-generation phase-transfer catalysts. Chem Rev 118(18):8770–8785
Zhao L, Hermann M, Holzmann N, Frenking G (2017) Dative bonding in main group compounds. Coord Chem Rev 344:163–204
Hansmann MM, Bertrand G (2016) Transition-metal-like behavior of main group elements: ligand exchange at a phosphinidene. J Am Chem Soc 138(49):15885–15888
Georgiou DC, Stringer BD, Hogan CF, Barnard PJ, Wilson DJ, Holzmann N, Frenking G, Dutton JL (2015) The fate of NHC-stabilized dicarbon. Chem Eur J 21(8):3377–3386
Rivard E (2014) Donor–acceptor chemistry in the main group. Dalton Trans 43(23):8577–8586
Himmel D, Krossing I, Schnepf A (2014) Dative bonds in main-group compounds: a case for fewer arrows! Angew Chem Int Ed 53(2):370–374
Frenking G (2014) Dative bonds in main-group compounds: a case for more arrows! Angew Chem Int Ed 53(24):6040–6046
Himmel D, Krossing I, Schnepf A (2014) Dative or not dative? Angew Chem Int Ed 53(24):6047–6048
Wilson DJ, Dutton JL (2013) Recent advances in the field of main-group mono-and diatomic “allotropes” stabilised by neutral ligands. Chem Eur J 19(41):13626–13637
Wilson DJ, Couchman SA, Dutton JL (2012) Are N-heterocyclic carbenes “better” ligands than phosphines in main group chemistry? A theoretical case study of ligand-stabilized E2 molecules, LEEL (L=NHC, phosphine; E=C, Si, Ge, Sn, Pb, N, P, As, Sb, Bi). Inorg Chem 51(14):7657–7668
Back O, Donnadieu B, von Hopffgarten M, Klein S, Tonner R, Frenking G, Bertrand G (2011) N-Heterocyclic carbenes versus transition metals for stabilizing phosphinyl radicals. Chem Sci 2(5):858–861
Singh T, Bharatam PV (2019) Donor→acceptor coordination interactions in 1,3-bis (NHC) triazenyl cations: an electronic structure analysis. J Comput Chem 40(25):2207–2215
Bharatam PV, Patel DS, Iqbal P (2005) Pharmacophoric features of biguanide derivatives: an electronic and structural analysis. J Med Chem 48(24):7615–7622
Patel DS, Bharatam PV (2009) Novel ⊕N(←L)2 species with two lone pairs on nitrogen: systems isoelectronic to carbodicarbenes. Chem Commun 9:1064–1066
Kozma Á, Gopakumar G, Farès C, Thiel W, Alcarazo M (2013) Synthesis and structure of carbene-stabilized N-centered cations [L2N]+, [L2NR]2+, [LNR3]2+, and [L3N]3+. Chem Eur J 19(11):3542–3546
Parr RG (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Hehre WJ, Radom L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Pople JA, Beveridge DL (1970) Approximate molecular orbital theory. McGraw-Hill Book, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Revision E.01 edn. Gaussian, Inc., Wallingford
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120(1–3):215–241
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J Org Chem 73(12):4615–4624
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92(9):5397–5403
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Ziegler T, Rauk A (1979) Carbon monoxide, carbon monosulfide, molecular nitrogen, phosphorus trifluoride, and methyl isocyanide as. sigma. donors and. pi. acceptors. A theoretical study by the Hartree-Fock-Slater transition-state method. Inorg Chem 18(7):1755–1759
Morokuma K (1971) Molecular orbital studies of hydrogen bonds. III. C=O···H–O hydrogen bond in H2CO···H2O and H2CO···2H2O. J Chem Phys 55(3):1236–1244
Mitoraj MP, Michalak A, Ziegler T (2009) A combined charge and energy decomposition scheme for bond analysis. J Chem Theory Comput 5(4):962–975
Mitoraj M, Michalak A (2008) Applications of natural orbitals for chemical valence in a description of bonding in conjugated molecules. J Mol Model 14(8):681–687
Michalak A, Mitoraj M, Ziegler T (2008) Bond orbitals from chemical valence theory. J Phys Chem A 112(9):1933–1939
Mitoraj M, Michalak A (2007) Donor–acceptor properties of ligands from the natural orbitals for chemical valence. Organometallics 26(26):6576–6580
Mitoraj M, Michalak A (2007) Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. J Mol Model 13(2):347–355
ADF2012, SCM, Theoretical Chemistry. Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com
Te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJ, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22(9):931–967
Herrmann WA (2002) N-heterocyclic carbenes: a new concept in organometallic catalysis. Angew Chem Int Ed 41(8):1290–1309
Peris E (2017) Smart N-heterocyclic carbene ligands in catalysis. Chem Rev 118(19):9988–10031
Patel N, Arfeen M, Sood R, Khullar S, Chakraborti AK, Mandal SK, Bharatam PV (2018) Can remote N-heterocyclic carbenes coordinate with main group elements? Synthesis, structure, and quantum chemical analysis of N+-centered complexes. Chem Eur J 24(24):6418–6425
Bharatam PV, Arfeen M, Patel N, Jain P, Bhatia S, Chakraborti AK, Khullar S, Gupta V, Mandal SK (2016) Design, synthesis, and structural analysis of divalent NI compounds and identification of a new electron-donating ligand. Chem Eur J 22(3):1088–1096
Bhatia S, Malkhede YJ, Bharatam PV (2013) Existence of dynamic tautomerism and divalent N (I) character in N-(pyridin-2-yl) thiazol-2-amine. J Comput Chem 34(18):1577–1588
Bhatia S, Bagul C, Kasetti Y, Patel DS, Bharatam PV (2012) Divalent N (I) character in 2-(thiazol-2-yl) guanidine: an electronic structure analysis. J Phys Chem A 116(36):9071–9079
Patel DS, Bharatam PV (2011) Divalent N(I) compounds with two lone pairs on nitrogen. J Phys Chem A 115(26):7645–7655
Patel N, Falke B, Bharatam PV (2018) C→N coordination bonds in (CCC)→N+←(L) complexes. Theor Chem Accounts 137(3):34
Celik MA, Sure R, Klein S, Kinjo R, Bertrand G, Frenking G (2012) Borylene complexes (BH) L2 and nitrogen cation complexes (N+) L2: isoelectronic homologues of carbones CL2. Chem Eur J 18(18):5676–5692
Tonner R, Frenking G (2008) Divalent carbon (0) chemistry, part 1: parent compounds. Chem Eur J 14(11):3260–3272
Bonn M, Hunger J (2021) Between a hydrogen and a covalent bond. Science 371(6525):123–124
Acknowledgments
Mr. Wanjari is grateful to National Institute of Pharmaceutical Education and Research (NIPER) S.A.S.-Nagar, India, for research facilities; Dr. Singh is thankful to the Department of Science and Technolgy (DST); and F.A. Sofi is thankful to National Institute of Pharmaceutical Education and Research (NIPER) S.A.S.-Nagar, for financial support.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1318 kb)
Rights and permissions
About this article
Cite this article
Wanjari, P.J., Singh, T., Sofi, F.A. et al. Quantum chemical study in exploring the role of donor→acceptor interactions in 1,3-bis carbene-stabilized guanidinium cations. J Mol Model 27, 87 (2021). https://doi.org/10.1007/s00894-021-04707-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04707-2