Abstract
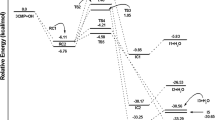
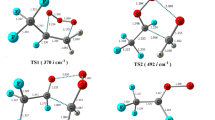
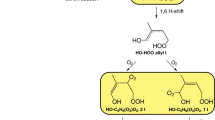
The formation of secondary organic aerosols caused by atmospheric oxidation of isoprene is harmful to human health and the climate; thus, isoprene oxidation is further mandatory to obtain less harmful or harmless highly oxidised products. In this numerical investigation, 2-hydroperoxy-2-methylbut-3-en-1-ol (ISOPOOH) was considered the model compound to investigate the formation of three RO2 radicals (C5H11O4, C5H11O6 and C5H11O5) and two saturated highly oxidised products (C5H12O6 and C5H10O6). The complete reaction network and its thermodynamics and kinetics were analysed to obtain the most probable and feasible reaction pathways. Four different levels of theories (HF, B3LYP, M06-2X and ωB97XD with basis set of 6-31+g(d,p)) were employed to explore a global minimum of ISOPOOH. All theories provided approximately close energetics; however, because of the novelty of the functional and parameterisation of the basis set, the ωB97XD functional was selected to examine the reaction mechanism. C5H12O6 was formed as the second-generation highly oxidised product during ISOPOOH oxidation.





Similar content being viewed by others
Data availability
Relevant data can be provided on request.
References
Harley PC, Monson RK, Lerdau MT (1999) Ecological and evolutionary aspects of isoprene emission from plants. Oecologia 118:109–123
Watson JG (2002) Visibility: science and regulation. J Air Waste Manage Assoc 52:628–713. https://doi.org/10.1080/10473289.2002.10470813
Pope CA, Dockery DW (2006) Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc 56:709–742. https://doi.org/10.1080/10473289.2006.10464485
Hallquist M, Wenger JC, Baltensperger U et al (2009) The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys 9:5155–5236. https://doi.org/10.5194/acp-9-5155-2009
Pandis SN, Paulson SE, Seinfeld JH, Flagan RC (1991) Aerosol formation in the photooxidation of isoprene and β-pinene. Atmos Environ Part A Gen Top 25:997–1008. https://doi.org/10.1016/0960-1686(91)90141-S
Glasius M, Goldstein AH (2016) Recent discoveries and future challenges in atmospheric organic chemistry. Environ Sci Technol 50:2754–2764. https://doi.org/10.1021/acs.est.5b05105
Guenther AB, Jiang X, Heald CL et al (2012) The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci Model Dev 5:1471–1492. https://doi.org/10.5194/gmd-5-1471-2012
Paulot F, Kjaergaard HG, Seinfeld JH et al (2009) Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science (80- ) 325:730–733. https://doi.org/10.1126/science.1172910
Ruppert L, Heinz Becker K (2000) A product study of the OH radical-initiated oxidation of isoprene: formation of C5-unsaturated diols. Atmos Environ 34:1529–1542. https://doi.org/10.1016/S1352-2310(99)00408-2
Berndt T, Hyttinen N, Herrmann H, Hansel A (2019) First oxidation products from the reaction of hydroxyl radicals with isoprene for pristine environmental conditions. Commun Chem 2:1–10. https://doi.org/10.1038/s42004-019-0120-9
Wennberg PO, Bates KH, Crounse JD et al (2018) Gas-phase reactions of isoprene and its major oxidation products. Chem Rev 118:3337–3390. https://doi.org/10.1021/acs.chemrev.7b00439
Bhuvaneswari R, Senthilkumar K (2019) Theoretical study on the gas phase reaction of methyl chavicol with hydroxyl radical. Comput Theor Chem 1151:78–90. https://doi.org/10.1016/j.comptc.2019.02.005
Paul S, Gour NK, Deka RC (2019) Mechanistic investigation of the atmospheric oxidation of bis(2-chloroethyl) ether (ClCH2CH2OCH2CH2Cl) by OH and NO3 radicals and cl atoms: a DFT approach. J Mol Model 25:43. https://doi.org/10.1007/s00894-019-3923-9
Cao H, Li X, He M, Zhao XS (2018) Computational study on the mechanism and kinetics of NO 3 -initiated atmosphere oxidation of vinyl acetate. Comput Theor Chem 1144:18–25. https://doi.org/10.1016/j.comptc.2018.09.012
Fan J, Zhang R (2006) Atmospheric oxidation mechanism of p-xylene: a density functional theory study. J Phys Chem A 110:7728–7737. https://doi.org/10.1021/jp061735e
So S, Wille U, Da Silva G (2015) A theoretical study of the photoisomerization of glycolaldehyde and subsequent OH radical-initiated oxidation of 1,2-ethenediol. J Phys Chem A 119:9812–9820. https://doi.org/10.1021/acs.jpca.5b06854
Thomas WC, Dresser WD, Cortés DA, Elrod MJ (2017) Gas phase oxidation of campholenic aldehyde and solution phase reactivity of its epoxide derivative. J Phys Chem A 121:168–180. https://doi.org/10.1021/acs.jpca.6b08642
Berndt T, Herrmann H, Sipilä M, Kulmala M (2016) Highly oxidized second-generation products from the gas-phase reaction of OH radicals with isoprene. J Phys Chem A 120:10150–10159. https://doi.org/10.1021/acs.jpca.6b10987
Ai Y, Liu Y, Huo Y et al (2019) Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ Sci Nano 6:3336–3348. https://doi.org/10.1039/c9en00866g
Wei D, Zhao C, Khan A et al (2019) Sorption mechanism and dynamic behavior of graphene oxide as an effective adsorbent for the removal of chlorophenol based environmental-hormones: a DFT and MD simulation study. Chem Eng J 375:121964. https://doi.org/10.1016/j.cej.2019.121964
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, revision B.01. Gaussian 09, Revis. B.01. Gaussian, Inc., Wallingford CT
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc., Shawnee Mission, KS
Becke AD (1993) Density-functional thermochemistry.III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/B810189B
Simón L, Goodman JM (2011) How reliable are DFT transition structures? Comparison of GGA, hybrid-meta-GGA and meta-GGA functionals. Org Biomol Chem 9:689–700. https://doi.org/10.1039/c0ob00477d
Hratchian HP, Schlegel HB (2004) Accurate reaction paths using a Hessian based predictor-corrector integrator. J Chem Phys 120:9918–9924. https://doi.org/10.1063/1.1724823
Verma AM, Agrawal K, Kawale HD, Kishore N (2018) Quantum chemical study on gas phase decomposition of ferulic acid. Mol Phys 116:1895–1907. https://doi.org/10.1080/00268976.2018.1464223
Materials availability
Relevant material can be provided on request.
Author information
Authors and Affiliations
Contributions
All authors are credited as per the author’s contributions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All ethical guidelines have been followed relevant to the preparation of this manuscript.
Consent for publication
All authors agree to the publication of the work.
Conflict of interest
The authors declare no competing interests.
Code availability
N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22.8 kb)
Rights and permissions
About this article
Cite this article
Verma, A.M., Singh, S.P. & Ojha, R.P. Quantum chemical study of gas-phase reactions of isoprene with OH radicals producing highly oxidised second-generation products. J Mol Model 27, 62 (2021). https://doi.org/10.1007/s00894-021-04666-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04666-8