Abstract
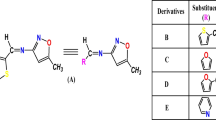
A series of twelve Acceptor-π-Donor-π-Acceptor (A-π-D-π-A) topology-based donor molecules, where diketopyrrolopyrrole (DPP) as donor core unit is connected through furan which acts as conjugated π-bridge (CB) to aromatic derivatives (Ar) as acceptor units, have been investigated by making substitutions in acceptor units by using density functional theory(DFT) and time-dependent density functional theory (TD-DFT) for organic solar cell applications. The comparative study of optoelectronic properties indicates that thiadiazole with pyridine units containing molecules (M6b) exhibit lower energy of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels than those of oxadiazole and pyridine containing units (M6b). Among our investigated donors, the smallest Eg of 1.60 eV was observed for both M6a and M6b with distinctive broad absorption at 843 and 857 nm, respectively. Overall, smaller electron transfer (λe) values in contrast to hole transfer (λh) demonstrate that these donor compounds would be best for λe. The calculated open circuit voltage (Voc) is 2.45 and 2.17 eV, regarding bisPCBM and PC60BM (phenyl-C61-butyric acid methyl ester) acceptors. Thus, these theoretical calculations not only endorse the deep consideration between the chemical structures and optoelectronic characteristics of the donor-acceptor systems but also suggest appropriate materials for high-performance Organic Photovoltaics (OPV).

Graphical abstract











Similar content being viewed by others
References
Irfan A, Al-Sehemi AG (2012) Quantum chemical study in the direction to design efficient donor-bridge-acceptor triphenylamine sensitizers with improved electron injection. J Mol Model 18(11):4893–4900
Nozik AJ, Miller J (2010) Introduction to solar photon conversion. ACS Publications, Washington, D.C.
Armaroli N, Balzani V (2007) The future of energy supply: challenges and opportunities. Angew Chem Int Ed 46(1–2):52–66
Shin WS et al (2006) Effects of functional groups at perylene diimide derivatives on organic photovoltaic device application. J Mater Chem 16(4):384–390
Lee JW et al (2016) Ternary blend composed of two organic donors and one acceptor for active layer of high-performance organic solar cells. ACS Appl Mater Interfaces 8(17):10961–10967
Tang CW (1986) Two-layer organic photovoltaic cell. Appl Phys Lett 48(2):183–185
Liang Y, Yu L (2010) Development of semiconducting polymers for solar energy harvesting. Polym Rev 50(4):454–473
Montcada NF et al (2013) High open circuit voltage in efficient thiophene-based small molecule solution processed organic solar cells. Org Electron 14(11):2826–2832
You J et al (2013) A polymer tandem solar cell with 10.6% power conversion efficiency. Nat Commun 4:1446
Zhou Y et al (2015) Synthesis and photovoltaic properties of new small molecules with rhodanine derivative as the end-capped blocks. Org Electron 17:355–363
Walker B, Kim C, Nguyen T-Q (2010) Small molecule solution-processed bulk heterojunction solar cells. Chem Mater 23(3):470–482
Sun Y et al (2012) Solution-processed small-molecule solar cells with 6.7% efficiency. Nat Mater 11(1):44
Welch GC et al (2011) A modular molecular framework for utility in small-molecule solution-processed organic photovoltaic devices. J Mater Chem 21(34):12700–12709
Sun Q et al (2015) Revealing the effect of donor/acceptor intermolecular arrangement on organic solar cells performance based on two-dimensional conjugated small molecule as electron donor. Org Electron 24:30–36
Blom PW et al (2007) Device physics of polymer: fullerene bulk heterojunction solar cells. Adv Mater 19(12):1551–1566
Lin Y, Li Y, Zhan X (2012) Small molecule semiconductors for high-efficiency organic photovoltaics. Chem Soc Rev 41(11):4245–4272
Wu F-C et al Influences of device structures on microstructure-correlated photovoltaic characteristics of organic solar cells. In Proc. of SPIE Vol. 2017
Kyaw AKK et al (2013) Efficient solution-processed small-molecule solar cells with inverted structure. Adv Mater 25(17):2397–2402
Zhou J et al (2013) Solution-processed and high-performance organic solar cells using small molecules with a Benzodithiophene unit. J Am Chem Soc 135(23):8484–8487
He C et al (2007) Synthesis and photovoltaic properties of a solution-Processable organic molecule containing Triphenylamine and DCM moieties. J Phys Chem C 111(24):8661–8666
Jin R, Chang Y (2015) A theoretical study on photophysical properties of triphenylamine-cored molecules with naphthalimide arms and different π-conjugated bridges as organic solar cell materials. Phys Chem Chem Phys 17(3):2094–2103
Kungwan N et al (2014) Theoretical study of linker-type effect in carbazole–carbazole-based dyes on performances of dye-sensitized solar cells. Theor Chem Accounts 133(8):1523
Martínez JP et al (2015) Extent of charge separation and exciton delocalization for electronically excited states in a triphenylamine-C60 donor–acceptor conjugate: a combined molecular dynamics and TD-DFT study. Theor Chem Accounts 134(2):12
Mohamad M et al (2015) First principles investigations of vinazene molecule and molecular crystal: a prospective candidate for organic photovoltaic applications. J Mol Model 21(2):27
Guo X et al (2012) Poly (thieno [3, 2-b] thiophene-alt-bithiazole): AD–A copolymer donor showing improved photovoltaic performance with indene-C60 bisadduct acceptor. Macromolecules 45(17):6930–6937
Roncali J (2009) Molecular bulk heterojunctions: an emerging approach to organic solar cells. Acc Chem Res 42(11):1719–1730
Demeter D et al (2011) Manipulation of the open-circuit voltage of organic solar cells by desymmetrization of the structure of acceptor–donor–acceptor molecules. Adv Funct Mater 21(22):4379–4387
Yin B et al (2010) Solution-processed bulk heterojunction organic solar cells based on an oligothiophene derivative. Appl Phys Lett 97(2):139
Zhang J et al (2011) Red-emission organic light-emitting diodes based on solution-processable molecules with triphenylamine core and benzothiadiazole-thiophene arms. SCIENCE CHINA Chem 54(4):695–698
Huang J et al (2012) Fine-tuning device performances of small molecule solar cells via the more polarized DPP-attached donor units. Phys Chem Chem Phys 14(41):14238–14242
Jin R, Wang K (2015) Rational design of diketopyrrolopyrrole-based small moleculesas donating materials for organic solar cells. Int J Mol Sci 16(9):20326–20343
Kim Y et al (2014) Synthesis of diketopyrrolopyrrole (DPP)-based small molecule donors containing thiophene or furan for photovoltaic applications. Mater Chem Phys 143(2):825–829
Miyata Y, Nishinaga T, Komatsu K (2005) Synthesis and structural, electronic, and optical properties of oligo(thienylfuran)s in comparison with oligothiophenes and oligofurans. J Organomet Chem 70(4):1147–1153
Frisch M et al (2009) Gaussian 09, revision D. 01. Gaussian, Inc, Wallingford
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170
Bibi S, Li P, Zhang J (2013) X-shaped donor molecules based on benzo[2,1-b:3,4-b′]dithiophene as organic solar cell materials with PDIs as acceptors. J Mater Chem A 1(44):13828
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65(3):599
Marcus RA (1964) Chemical and electrochemical electron-transfer theory. Annu Rev Phys Chem 15(1):155–196
Prezhdo OV (2002) Assessment of theoretical approaches to the evaluation of dipole moments of chromophores for nonlinear optics. Adv Mater 14(8):597–600
Köse ME et al (2007) Theoretical studies on conjugated phenyl-cored thiophene dendrimers for photovoltaic applications. J Am Chem Soc 129(46):14257–14270
Chen JD et al (2015) Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv Mater 27(6):1035–1041
Bai H et al (2014) Acceptor–donor–acceptor small molecules based on indacenodithiophene for efficient organic solar cells. ACS Appl Mater Interfaces 6(11):8426–8433
Lenes M et al (2008) Fullerene bisadducts for enhanced open-circuit voltages and efficiencies in polymer solar cells. Adv Mater 20(11):2116–2119
Shaheen SE et al (2001) 2.5% efficient organic plastic solar cells. Appl Phys Lett 78(6):841–843
Wienk MM et al (2003) Efficient methano [70] fullerene/MDMO-PPV bulk heterojunction photovoltaic cells. Angew Chem 115(29):3493–3497
Qin C et al (2011) Quantum chemical study of structures, electronic spectrum, and nonlinear optical properties of polynuclear lithium compounds. Comput Theor Chem 966(1):14–19
Gruhn NE et al (2002) The vibrational reorganization energy in pentacene: molecular influences on charge transport. J Am Chem Soc 124(27):7918–7919
Lin BC et al (2005) Charge transport properties of tris (8-hydroxyquinolinato) aluminum (III): why it is an electron transporter. J Am Chem Soc 127(1):66–67
Tang S, Zhang J (2011) Rational design of organic asymmetric donors D1-A-D2 possessing broad absorption regions and suitable frontier molecular orbitals to match typical acceptors toward solar cells. J Phys Chem A 115(20):5184–5189
Martin RL (2003) Natural transition orbitals. J Chem Phys 118(11):4775–4777
Acknowledgments
The calculations were performed in the computational and theoretical chemistry laboratory at department of chemistry, University of Agriculture Faisalabad, Pakistan. The authors vastly acknowledge the Higher education commission (HEC) of Pakistan and University of Agriculture Faisalabad for financial and technical support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3379 kb).
Rights and permissions
About this article
Cite this article
Shafiq UrRehman, Alam, A., Bibi, S. et al. The effect of different aromatic conjugated bridges on optoelectronic properties of diketopyrrolopyrrole-based donor materials for organic photovoltaics. J Mol Model 26, 154 (2020). https://doi.org/10.1007/s00894-020-4341-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4341-8