Abstract
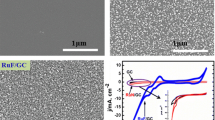
In recent years, nicotinamide adenine dinucleotide (NADH) and its oxidized form (NAD+) have withdrawn a substantial attention since they possess a significant place in both biosensor and biofuel cell studies. However, the transformation of NADH to NAD+ brings about the surface passivation and fouling at the most of corresponding conductive materials; consequently, significant decrease takes place in the current. In order to overcome these drawbacks, we have performed the surface functionalization of single-walled carbon nanotube (SWCNT) and graphene oxide (GO) immobilized onto glassy carbon surface with dihydroxybenzene (di-HB) using solid-phase synthesis methodology. The di-HB-modified SWCNT and GO were found to exhibit great catalytic activity as they reduce required overpotential of electrochemical oxidation of NADH and lead to enhancement in the peak current, compared with unmodified carbon electrodes. Molecular docking simulation technique was also carried out to enlighten attained experimental findings in detail, and we have found that increase in the binding affinity of NAD+ to functionalized carbon surfaces with di-HB is related to formation of hydrogen bonding interactions Furthermore, our experimental and theoretical outputs were also found to be quite consistent in terms of reactivity of modified surfaces to NADH oxidation.





Similar content being viewed by others
References
Gorton L (1986) Chemically modified electrodes for the electrocatalytic oxidation of nicotinamide coenzymes. J Chem Soc, Faraday Trans 1: Phys Chem Condens Phases 82:1245–1258. https://doi.org/10.1039/F19868201245
Gorton L, Domınguez E (2002) Electrocatalytic oxidation of NAD(P)H at mediator-modified electrodes. Rev Mol Biotechnol 82:371–392. https://doi.org/10.1016/S1389-0352(01)00053-8
Bartlett PN (2008) Bioelectrochemistry: fundamentals, experimental techniques and applications. John Wiley & Sons, Chichester
Banks CE, Compton RG (2005) Exploring the electrocatalytic sites of carbon nanotubes for NADH detection: an edge plane pyrolytic graphite electrode study. Analyst 130:1232–1239. https://doi.org/10.1039/B508702C
Gao F, Yan Y, Su L, Wang L, Mao L (2007) An enzymatic glucose/O2 biofuel cell: preparation, characterization and performance in serum. Electrochem Commun 9:989–996. https://doi.org/10.1016/j.elecom.2006.12.008
Pérez B, del Valle M, Alegret S, Merkoçi A (2007) Carbon nanofiber vs. carbon microparticles as modifiers of glassy carbon and gold electrodes applied in electrochemical sensing of NADH. Talanta 74:398–404. https://doi.org/10.1016/j.talanta.2007.10.022
Pumera M (2009) The electrochemistry of carbon nanotubes: fundamentals and applications. Chem Eur J 15:4970–4978. https://doi.org/10.1002/chem.200900421
Pumera M, Scipioni R, Iwai H, Ohno T, Miyahara Y, Boero M (2009) A mechanism of adsorption of β-nicotinamide adenine dinucleotide on graphene sheets: experiment and theory. Chem Eur J 15:10851–10856. https://doi.org/10.1002/chem.200900399
Ghanem MA, Chrétien J-M, Kilburn JD, Bartlett PN (2009) Electrochemical and solid-phase synthetic modification of glassy carbon electrodes with dihydroxybenzene compounds and the electrocatalytic oxidation of NADH. Bioelectrochem 76:115–125. https://doi.org/10.1016/j.bioelechem.2009.02.008
Maleki A, Nematollahi D, Clausmeyer J, Henig J, Plumeré N, Schuhmann W (2012) Electrodeposition of catechol on glassy carbon electrode and its electrocatalytic activity toward NADH oxidation. Electroanal 24:1932–1936. https://doi.org/10.1002/elan.201200251
Matsue T, Suda M, Uchida I, Kato T, Akiba U, Osa T (1987) Electrocatalytic oxidation of NADH by ferrocene derivatives and the influence of cyclodextrin complexation. J Electroanal Chem Interfacial Electrochem 234:163–173. https://doi.org/10.1016/0022-0728(87)80169-9
Pinczewska A, Sosna M, Bloodworth S, Kilburn JD, Bartlett PN (2012) High-throughput synthesis and electrochemical screening of a library of modified electrodes for NADH oxidation. J Am Chem Soc 134:18022–18033. https://doi.org/10.1021/ja307390x
Scipioni R, Pumera M, Boero M, Miyahara Y, Ohno T (2010) Investigation of the mechanism of adsorption of β-nicotinamide adenine dinucleotide on single-walled carbon nanotubes. J Phys Chem Lett 1:122–125. https://doi.org/10.1021/jz9000714
Chrétien J-M, Ghanem MA, Bartlett PN, Kilburn JD (2008) Covalent tethering of organic functionality to the surface of glassy carbon electrodes by using electrochemical and solid-phase synthesis methodologies. Chem Eur J 14:2548–2556. https://doi.org/10.1002/chem.200701559
Ghanem MA, Chrétien J-M, Pinczewska A, Kilburn JD, Bartlett PN (2008) Covalent modification of glassy carbon surface with organic redox probes through diamine linkers using electrochemical and solid-phase synthesis methodologies. J Mater Chem 18:4917–4927. https://doi.org/10.1039/B809040H
Ghanem MA, Kocak I, Al-Mayouf A, AlHoshan M, Bartlett PN (2012) Covalent modification of carbon nanotubes with anthraquinone by electrochemical grafting and solid phase synthesis. Electrochim Acta 68:74–80. https://doi.org/10.1016/j.electacta.2012.02.027
Ghanem MA, Kocak I, Al-Mayouf A, Bartlett PN (2013) Solid phase modification of carbon nanotubes with anthraquinone and nitrobenzene functional groups. Electrochem Commun 34:258–262. https://doi.org/10.1016/j.elecom.2013.05.039
Manikandan A, Sathiyabama M (2015) Green synthesis of copper-chitosan nanoparticles and study of its antibacterial activity. J Nanomed Nanotech 6:1–4. https://doi.org/10.4172/2157-7439.1000251
Shahriary L, Athawale A (2014) Graphene oxide synthesized by using modified hummers approach. Int J Renew Energy Environ Eng 2:58–63
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17. https://doi.org/10.1186/1758-2946-4-17
Delano WL (2002) The PyMOL Molecular Graphics System. http://www.pymol.org
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Solis FJ, Wets RJ-B (1981) Minimization by random search techniques. J Math Oper Res 6:19–30. https://doi.org/10.1287/moor.6.1.19
Biovia DS (2017) Discovery studio modeling environment. Dassault Systèmes, San Diego
Chrétien J-M, Ghanem MA, Bartlett PN, Kilburn JD (2009) Covalent modification of glassy carbon surfaces by using electrochemical and solid-phase synthetic methodologies: application to bi- and trifunctionalisation with different redox centres. Chem Eur J 15:11928–11936. https://doi.org/10.1002/chem.200901135
Baez DF, Tapia F, Sierra-Rosales P, Bollo S (2018) In situ electroreduction of graphene oxide: increased sensitivity for the determination of NADH. Electroanalysis 30:1–8. https://doi.org/10.1002/elan.201800629
Acknowledgments
The authors thank Zonguldak Bülent Ecevit University Faculty of Pharmacy and Science and Art for allowing to use facilities of faculties.
Funding
This study received financial support from Zonguldak Bülent Ecevit University Faculty of Pharmacy and Science and Art.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 864 kb)
Rights and permissions
About this article
Cite this article
Koçak, İ., Alıcı, H. Experimental and theoretical studies of electrochemical oxidation of nicotinamide adenine dinucleotide at the modified SWCNT and graphene oxide. J Mol Model 26, 51 (2020). https://doi.org/10.1007/s00894-020-4314-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4314-y