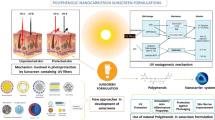
Abstract
Sunscreen-based photoprotection is an important strategy to prevent photoaging and skin cancer. Among the effective and modern sunscreens, triazine compounds are known as an important class based on their physical-chemical properties, such as photostability and UV broad-spectrum absorption (UVA and UVB). Molecular modeling and quantum mechanical calculations approaches can be helpful to orientate the design of sunscreens. Herein, a case study is presented to demonstrate the importance of the molecular modeling as a design tool for promising sunscreen candidates based on the synthesis research previously described of bemotrizinol, a broad-spectrum photostable organic UV filter present in many sunscreens products. Time-dependent density functional theory (TD-DFT) calculations performed in gas phase on the isolated organic UV filters proved to reproduce the experimental UV absorption, guiding the choice of the most efficient candidate as sunscreen. The present work highlights the importance of molecular modeling as an effective tool to support synthesis research, increasing the possibility of obtaining promising compounds with reduced costs and effluent production.

A case study to demonstrate the importance of the molecular modeling as a design tool for promising sunscreen candidates is presented. The method proved to be a valuable tool to reproduce the experimental UV absorption and to determinate the most efficient molecule as sunscreen among the candidates.







Similar content being viewed by others
Change history
06 January 2020
The original version of this article unfortunately contained mistakes. Table 1 was missing and the presentation of Table 2 was incorrect. In Table 2, the second [λexp (nm)] and last [MADa] columns, many values are wrongly in the same cell/line. For example, in column 2, line 2, the first number (342) should be above the other (318).
Abbreviations
- UVR, UV:
-
ultraviolet radiation
- ROS:
-
reactive oxygen species
- TD-DFT:
-
time-dependent density functional theory
- PM6:
-
parametric method 6
- BEMT:
-
bemotrizinol
- MAD:
-
mean absolute deviation
- B3LYP:
-
Becke 3-parameter Lee-Yang-Parr
References
World Health Organization. http://www.who.int/uv/faq/skincancer/en/index1.html (accessed December 1, 2018)
Diepgen TL, Fartasch M, Drexler H, Schmitt J (2012) Occupational skin cancer induced by ultraviolet radiation and its prevention. Br J Dermatol 167:76–84
Madan V, Lear JT, Szeimies RM (2010) Non-melanoma skin cancer. Lancet 375(9715):673–685
Stavros VG (2014) A bright future for sunscreens. Nat Chem 6:955–956
Koh HK, Geller AC, Miller DR et al (1996) Prevention and early detection strategies for melanoma and skin cancer: current status. Arch Dermatol 132(4):436–442
Parkin DM, Mesher D, Sasieni P (2011) Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer 105:S66–S69
Rodrigues NN, Stavros VG (2018) From fundamental science to product: a bottom-up approach. Sci Prog 101(1):8–31
Hussein MR (2005) Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutan Pathol 32:191–205
Matsumura Y, Ananthaswamy HN (2004) Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 195:298–308
Krutmann J (2001) The role of UVA rays in skin aging. Eur J Dermatol 11:170–171
Montagner S, Costa A (2009) Bases biomoleculares do fotoenvelhecimento. Anais Brasileiros de Dermatolologia 84:263–269
Baker LA et al (2017) Photoprotection: extending lessons learned from studying natural sunscreens to the design of artificial sunscreen constituents. Chem Soc Rev 46:3770
Wang SQ et al (2010) Photoprotection: a review of the current and future technologies. Dermatol Ther 23:31–47
Dummer R, Maier T (2002) UV protection and skin cancer. Cancers of the Skin:7–12
Chatelain E et al (2001) Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochem Photobiol 74:401–406
Shaath NA (2010) Ultraviolet filters. Photochem Photobiol Sci 9:464–469
Herzog B, Hüglin D, Borsos E, Stehlin A, Luther H (2004) New UV absorbers for cosmetic sunscreens: a breakthrough for the photoprotection of human skin. Chimia International Journal of Chemistry 58:554–559
Couteau C, Paparis E, Chauvet C, Coiffard L (2015) Tris-biphenyl triazine, a new ultraviolet filter studied in terms of photoprotective efficacy. Int J Pharm 487:120–123
Corrêa BA, Gonçalves AS, de Souza AM et al (2012) Molecular modeling studies of the structural, electronic, and UV absorption properties of benzophenone derivatives. J Phys Chem A 116(45):10927–10933
Santos BA, Silva ACP, Bello M et al (2018) Molecular modeling for the investigation of UV absorbers for sunscreens: triazine and benzotriazole derivatives. J Photochem Photobiol A Chem 356:219–229
Walters C, Keeney A, Wigal CT et al (1997) The spectrophotometric analysis and modeling of sunscreens. J Chem Educ 74(1):99
Amat A, Clementi C, De Angelis F et al (2009) Absorption and Emission of the Apigenin and Luteolin Flavonoids: A TDDFT Investigation. J Phys Chem A 113:15118–15126
Liu F, Du L, Lan Z, Gao J (2017) Hydrogen bond dynamics governs the effective photoprotection mechanism of plant phenolic sunscreens. Photochem Photobiol Sci 15;16(2):211–219
Prommin C, Kanlayakan N, Chansen W et al (2017) Theoretical insights on solvent control of intramolecular and intermolecular proton transfer of 2-(2′Hydroxyphenyl) benzimidazole. J Phys Chem A 121(31):5773–5784
Pijeau S, Foster D, Hohenstein EG (2017) Excited-state dynamics of a benzotriazole photostabilizer: 2-(2′- Hydroxy-5′-methylphenyl) benzotriazole. J Phys Chem A 121:6377–6387
Zhao Y, Yang Y (2016) Excited state proton transfer coupled with twisted intermolecular charge transfer for N,N-dimethylanilino-1,3-diketone in high polar acetonitrile solvent. J Mol Liq 220:735–741
Marchetti B, Karsili TN (2016) Theoretical insights into the photo-protective mechanisms of natural biological sunscreens: building blocks of eumelanin and pheomelanin. Phys Chem Chem Phys 18:3644
Fang Z, Wu F, Tao Q et al (2019) Substituent effects on the ultraviolet absorption properties of stilbene compounds—models for molecular cores of absorbents. Spectrochim Acta A Mol Biomol Spectrosc 215:9–14
Silva ACP, Paiva JP, Diniz RR, dos Anjos VM et al (2019) Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein : in vitro and in silico approach for improved sunscreens. J Photochem Photobiol B Biol 193:162–171
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519
Stewart JJ (2007) Optimization of parameters for semi-empirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem Phys Lett 157:200–206
Lee C, Yan W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Kutzke H, Klapper H, Hammond RB, Roberts KJ (2000) Metastable beta-phase of benzophenone: independent structure determinations via X-ray powder diffraction and single crystal studies. Acta Crystallographica Section B 56:486–496
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST et al (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Jacquemin D, Perpete EA, Ciofini I, Adamo C (2008) Accurate simulation of optical properties in dyes. Acc Chem Res 42(2):326–334
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Acknowledgments
The authors would like to acknowledge the contribution to this paper from the undergraduate students Lucas Pereira Marques and Carolina Jardim Martins for helping with the data collection.
Funding
This study was supported by grants and fellowships from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro), and UFRJ (Universidade Federal do Rio de Janeiro), Brazil.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Table 1 was missing and the presentation of Table 2 was incorrect. In Table 2, second [λexp (nm)] and last [MADa] columns, many values are wrongly in the same cell/line.
Electronic supplementary material
ESM 1
(DOCX 299 kb)
Rights and permissions
About this article
Cite this article
Teixeira Gomes, J.V., Cherem Peixoto da Silva, A., Lamim Bello, M. et al. Molecular modeling as a design tool for sunscreen candidates: a case study of bemotrizinol. J Mol Model 25, 362 (2019). https://doi.org/10.1007/s00894-019-4237-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4237-7