Abstract
The solvation of mercury and halogens ions in water is essential for studying the reaction kinetics of various mercury depletion reactions in the atmosphere. Here, we use two approaches. The first one is the implementation of transition state theory to study the recombination reactions of Hg2+and Hal− with the introduction of a water molecule as a third body part. The inclusion of solvation corrections to the total energy enables one to localize the barrier for such diatomic systems with explicit water molecule participation. The second approach is the molecular modeling of three mercuric halide ion pairs in water complexes [HgHal(H2O)n]+ (Hal = Cl, Br, I) by using the semiempirical tight-binding molecular dynamics combined with density functional theory calculations. Various [Hg-Hal]+ ion pairs behave similarly when hydrated and tend to adopt clathrate-like configurations with a [Hg2+(H2O)6] central motif and halogen ions residing on the external surface of the water complex. Contact ion pairs are energetically favorable for all complexes up to 50 water molecules. Further increase in the level of hydration stabilized the solvent-separated forms of [Hg-Hal]+ ion pairs, which matches the water affinity rule. The balance between the contact and the solvated ion pairs was shown to be ion-pair specific and temperature dependent.

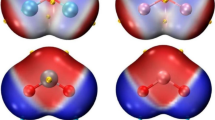
The structure of stable water complexes of mercury halides reflects the competition between water-water, Hg2+ -water, and Hal -water interactions.






Similar content being viewed by others
References
Ariya P. A., Peterson K., Snider, G. & Amyot, M. Mercury chemical transformations in the gas, aqueous and heterogeneous phases: state-of-the-art science and uncertainties. in Mercury Fate and Transport in the Global Atmosphere 459–501 (Springer US, 2009). https://doi.org/10.1007/978-0-387-93958-2_15
Lin C., Singhasuk, P. & Pehkonen S. Environmental chemistry and toxicology of mercury. (John Wiley & Sons, Inc., 2012)
Spicer CW et al (2002) Molecular halogens before and during ozone depletion events in the Arctic at polar sunrise: concentrations and sources. Atmos. Environ. 36:2721–2731
Barrie LA, Bottenheim JW, Schnell RC, Crutzen PJ, Rasmussen RA (1988) Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature 334:138–141
Boudries H, Bottenheim JW (2000) Cl and Br atom concentrations during a surface boundary layer ozone depletion event in the Canadian High Arctic. Geophys. Res. Lett. 27:517–520
Lindberg SE et al (2002) Dynamic oxidation of gaseous mercury in the Arctic troposphere at polar sunrise. Environ. Sci. Technol. 36:1245–1256
Temme C, Einax JW, Ebinghaus R, Schroeder WH (2003) Measurements of atmospheric mercury species at a coastal site in the Antarctic and over the South Atlantic Ocean during polar summer. Environ. Sci. Technol. 37:22–31
Skov H et al (2004) Fate of elemental mercury in the Arctic during atmospheric mercury depletion episodes and the load of atmospheric mercury to the Arctic. Environ. Sci. Technol. 38:2373–2382
Gauchard P et al (2005) Study of the origin of atmospheric mercury depletion events recorded in Ny-Ålesund, Svalbard, spring 2003. Atmos. Environ. 39:7620–7632
Lu JY et al (2001) Magnification of atmospheric mercury deposition to polar regions in springtime: the link to tropospheric ozone depletion chemistry. Geophys. Res. Lett. 28:3219–3222
Lin CJ, Pehkonen SO (1998) Two-phase model of mercury chemistry in the atmosphere. Atmos Env. 32:2543–2558
Hynes A. J., Donohoue D. L., Goodsite M. E. & Hedgecock I.M.. Our current understanding of major chemical and physical processes affecting mercury dynamics in the atmosphere and at the air-water/terrestrial interfaces. in Mercury fate and transport in the global atmosphere 427–457 (Springer, 2009)
Castro L, Dommergue A, Ferrari C, Maron L (2009) A DFT study of the reactions of O-3 with Hg degrees or Br. Atmos. Environ. 43:5708–5711
Khalizov A, Viswanathan B, Larregaray P, Ariya P (2003) A theoretical study on the reactions of Hg with halogens: atmospheric implications. J. Phys. Chem. A 107:6360–6365
Munthe J (1992) The aqueous oxidation of elemental mercury by ozone. Atmos Env. A 26:1461–1468
Lin CJ, Pehkonen SO (1997) Aqueous free radical chemistry of mercury in the presence of iron oxides and ambient aerosol. Atmos Env. 31:4125–4137
Wang Z, Pehkonen SO (2004) Oxidation of elemental mercury by aqueous bromine: atmospheric implications. Atmos Env. 38:3675–3688
Holmes CD, Jacob DJ, Yang X (2006) Global lifetime of elemental mercury against oxidation by atomic bromine in the free troposphere. Geophys. Res. Lett. 33(5)
Shepler BC, Wright AD, Balabanov NB, Peterson KA (2007) Aqueous microsolvation of mercury halide species. J. Phys. Chem. A 111:11342–11349
Maron L, Dommergue A, Ferrari C, Delacour-Larose M, Faïn X (2008) How elementary mercury reacts in the presence of halogen radicals and/or halogen anions: a DFT investigation. Chem. - A Eur. J. 14:8322–8329
Majumdar D, Roszak S, Leszczynski J (2011) Probing the structures and thermodynamic characteristics of the environment polluting mercuric halides, cyanides and thiocyanates. Chem. Phys. Lett. 501:308–314
Liu C-W et al (2014) Stable salt–water cluster structures reflect the delicate competition between ion–water and water–water interactions. J. Phys. Chem. B 118:743–751
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements ( Z = 1–86). J. Chem. Theory Comput. 13:1989–2009
Frisch, M. J. et al. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT. (2009)
Song Y, Akin-Ojo O, Wang F (2010) Correcting for dispersion interaction and beyond in density functional theory through force matching. J. Chem. Phys. 133:174115
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 3:214–218
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 17:49–56
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Accounts 120:215–241
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem 24:669–681
Marcus Y (2009) Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 109:1346–1370
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc., Faraday Trans 87:2995–2999
Collins KD (1997) Charge density-dependent strength of hydration and biological structure. Biophys. J. 72:65–76
Acknowledgments
U.S. Army Research Office ARO W911NF-16-1-0486 supported this work. We thank the Mississippi Center for Supercomputer Research (Oxford, MS, USA) for an allotment of computer time.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the Topical Collection Tim Clark 70th Birthday Festschrift
Electronic supplementary material
ESM 1
(DOCX 399 kb)
Rights and permissions
About this article
Cite this article
Zubatiuk, T., Hill, G. & Leszczynski, J. How water affects mercury–halogen interaction in the atmosphere. J Mol Model 25, 357 (2019). https://doi.org/10.1007/s00894-019-4212-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4212-3