Abstract
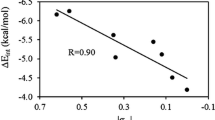
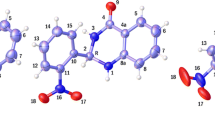
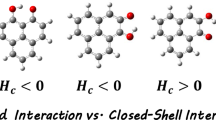
Quantum chemical calculations have been performed to gauge intramolecular hydrogen bonding and π-π stacking interactions of 8-hydroxyquinoline (8-HQ) derivatives (substituents X = OH, CH3, H, F, Cl, CF3, CN, NO2) supermolecular structures. The effects of substituents on 8-HQ derivatives have also been studied with the M06-2X method and 6-311++G** basis set. The basis set superposition error (BSSE)-corrected binding energies (ΔE), hydrogen bonding distances (rH···N), and intermolecular distance (d┻) were calculated with corresponding Hammett electronic parameters (σ). The topological parameters (ρ, ∇ρ2) and energy of intramolecular hydrogen bonding interaction (EH···N) of these π-π stacking 8-HQ derivatives have been studied by using atoms in molecules (AIM) theory. The electrostatic potential (ESP) analysis has been applied to gain the most negative and the most positive electrostatic potential V(r) values (Vs,min and Vs,max). It can be found that there are good relationships between the Hammett constants and the Vs,min values.






Similar content being viewed by others
References
Richter MM (2004) Electrochemiluminescence (ecl)[J]. Chem Rev 104(6):3003–3036
Prodi L, Bargossi C, Montalti M et al (2000) An effective fluorescent chemosensor for mercury ions [J]. J Am Chem Soc 122(28):6769–6770
Pearce D. A., Jotterand N., Carrico I. S., et al. Derivatives of 8-hydroxy-2-methylquinoline are powerful prototypes for zinc sensors in biological systems [J]. J Am Chem Soc, 2001, 123(21): 5160–5161
Pierre J, Baret P, Serratrice G (2003) Hydroxyquinolines as iron chelators [J]. Curr Med Chem 10(12):1077–1084
Zouhiri F, Danet M, Bénard C et al (2005) HIV-1 replication inhibitors of the styrylquinoline class: introduction of an additional carboxyl group at the C-5 position of the quinoline [J]. Tetrahedron Lett 46(13):2201–2205
Palit P, Paira P, Hazra A et al (2009) Phase transfer catalyzed synthesis of bis-quinolines: antileishmanial activity in experimental visceral leishmaniasis and in vitro antibacterial evaluation [J]. Eur J Med Chem 44(2):845–853
Musiol R, Jampilek J, Buchta V et al (2006) Antifungal properties of new series of quinoline derivatives [J]. Bioorg Med Chem 14(10):3592–3598
Roth HJ, Fenner H (2000) Arzneistoffe, 3rd ed. Deutscher Apotheker Verlag, Stuttgart, pp 51–114
Barnham K, Gautier E, Kok G et al. (2004) 8-Hydroxy quinoline derivatives, PCT Patent [J]. WO2004/007461
Zeng HP, Wang TT, Ouyang XH et al (2006) 8-Hydroxyquinoline derivatives induce the proliferation of rat mesenchymal stem cells (rMSCs)[J]. Bioorg Med Chem 14(16):5446–5450
Cai W, Hassani M, Karki R et al (2010) Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumor agents [J]. Bioorg Med Chem 18(5):1899–1909
Kakadiya R, Dong H, Kumar A et al (2010) Potent DNA-directed alkylating agents: synthesis and biological activity of phenyl N-mustard-quinoline conjugates having a urea or hydrazinecarboxamide linker [J]. Bioorg Med Chem 18(6):2285–2299
Kategaonkar AH, Shinde PV, Kategaonkar AH et al (2010) Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl) quinoline derivatives via click chemistry approach [J]. Eur J Med Chem 45(7):3142–3146
Beauchard A, Jaunet A, Murillo L et al (2009) Synthesis and antitumoral activity of novel thiazolobenzotriazole, thiazoloindolo [3, 2-c] quinoline and quinolinoquinoline derivatives [J]. Eur J Med Chem 44(10):3858–3865
Lehn JM (1995) Supramolecular chemistry [M]. Vch, Weinheim, Germany
Philp D, Stoddart JF (1996) Self-assembly in natural and unnatural systems [J]. Angew Chem Int Ed Eng 35(11):1154–1196
Lindsey JS (1991) Self-assembly in synthetic routes to molecular devices. Biological principles and chemical perspectives: a review [J]. New J Chem 15(2–3):153–180
Ebrahimi A, Habibi-Khorassani M, Gholipour AR et al (2009) Interaction between uracil nucleobase and phenylalanine amino acid: the role of sodium cation in stacking [J]. Theor Chem Accounts 124(1–2):115–122
Versees W, Loverix S, Vandemeulebroucke A et al (2004) Leaving group activation by aromatic stacking: an alternative to general acid catalysis [J]. J Mol Biol 338(1):1–6
Busker M, Svartsov YN, Häber T et al (2009) IR-UV double resonance spectra of pyrazine dimers: competition between CH⋯ π, π⋯ π and CH⋯ N interactions [J]. Chem Phys Lett 467(4–6):255–259
Sinnokrot MO, Sherrill CD (2003) Unexpected substituent effects in face-to-face π-stacking interactions [J]. J Phys Chem A 107(41):8377–8379
Zaccheddu M, Filippi C, Buda F (2008) Anion-π and π-π cooperative interactions regulating the self-assembly of nitrate-triazine-triazine complexes [J]. J Phys Chem A 112(7):1627–1632
Alty IG, Cheek DW, Chen T et al (2016) Intramolecular hydrogen-bonding effects on the fluorescence of PRODAN derivatives [J]. J Phys Chem A 120(20):3518–3523
Lane JR, Schrøder SD, Saunders GC et al (2016) Intramolecular hydrogen bonding in substituted aminoalcohols [J]. J Phys Chem A 120(32):6371–6378
Das P, Das PK, Arunan E (2015) Conformational stability and intramolecular hydrogen bonding in 1, 2-ethanediol and 1, 4-butanediol [J]. J Phys Chem A 119(16):3710–3720
Schrøder SD, Wallberg JH, Kroll JA et al (2015) Intramolecular hydrogen bonding in methyl lactate [J]. J Phys Chem A 119(37):9692–9702
Wang W, Yu L (2011) Intramolecular hydrogen bonding assisted charge transport through single rectifying molecule [J]. Langmuir 27(6):2084–2087
Estarellas C, Frontera A, Quiñonero D et al (2009) Interplay between cation-π and hydrogen bonding interactions: are non-additivity effects additive?[J]. Chem Phys Lett 479(4–6):316–320
Vijay D, Zipse H, Sastry GN (2008) On the cooperativity of cation-π and hydrogen bonding interactions [J]. J Phys Chem B 112(30):8863–8867
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions [J]. J Chem Theory Comput 2(2):364–382
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals [J]. Theor Chem Accounts 120(1–3):215–241
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry [J]. Acc Chem Res 41(2):157–167
Zhao Y, Truhlar DG (2008) Benchmark data for interactions in zeolite model complexes and their use for assessment and validation of electronic structure methods [J]. J Phys Chem C 112(17):6860–6868
Hohenstein EG, Chill ST, Sherrill CD (2008) Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules [J]. J Chem Theory Comput 4(12):1996–2000
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors [J]. Mol Phys 19(4):553–566
Koopmans T (1933). Physica 1:104
Parr RG, Szentpály L, Liu S (1999) Electrophilicity index [J]. J Am Chem Soc 121(9):1922–1924
Mortier WJ, Ghosh SK, Shankar S (1986) Electronegativity-equalization method for the calculation of atomic charges in molecules [J]. J Am Chem Soc 108(15):4315–4320
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer [J]. J Comput Chem 33(5):580–592
Fedichev PO, Reynolds MW, Shlyapnikov GV (1996) Three-body recombination of ultracold atoms to a weakly bound s level [J]. Phys Rev Lett 77(14):2921
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint [J]. Chem Rev 88(6):899–926
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics [J]. J Mol Graph 14(1):33–38
Frisch M, Trucks GW, Schlegel HB et al. (2009) Gaussian 09, revision E. 01[J].
Hammett LP (1935) Some relations between reaction rates and equilibrium constants [J]. Chem Rev 17(1):125–136
Zhu W, Tan X, Shen J et al (2003) Differentiation of cation-π bonding from cation-π intermolecular interactions: a quantum chemistry study using density-functional theory and Morokuma decomposition methods [J]. J Phys Chem A 107(13):2296–2303
Matta CF, Castillo N, Boyd RJ (2006) Extended weak bonding interactions in DNA: π-stacking (base-base), base-backbone, and backbone-backbone interactions [J]. J Phys Chem B 110(1):563–578
Zhikol OA, Shishkin OV, Lyssenko KA et al (2005) Electron density distribution in stacked benzene dimers: a new approach towards the estimation of stacking interaction energies [J]. J Chem Phys 122(14):144104
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities [J]. Chem Phys Lett 285(3–4):170–173
Alcami M., Mó O., Yanez M.. Modelling intrinsic basicities: the use of the electrostatic potentials and the atoms-in-molecules theory [M] Theoretical and Computational Chemistry. Elsevier, 1996, 3: 407–456
Suresh CH (2006) Molecular electrostatic potential approach to determining the steric effect of phosphine ligands in organometallic chemistry [J]. Inorg Chem 45(13):4982–4986
Murray JS, Ranganathan S, Politzer P (1991) Correlations between the solvent hydrogen bond acceptor parameter. beta. and the calculated molecular electrostatic potential [J]. J Org Chem 56(11):3734–3737
Murray JS, Politzer P (1991) Correlations between the solvent hydrogen-bond-donating parameter. Alpha. And the calculated molecular surface electrostatic potential [J]. J Org Chem 56(23):6715–6717
Hagelin H, Murray JS, Politzer P et al (1995) Family-independent relationships between computed molecular surface quantities and solute hydrogen bond acidity/basicity and solute-induced methanol O-H infrared frequency shifts [J]. Can J Chem 73(4):483–488
Hunter CA (2004) Quantifying intermolecular interactions: guidelines for the molecular recognition toolbox [J]. Angew Chem Int Ed 43(40):5310–5324
Aakeröy CB, Epa K, Forbes S et al (2013) Ranking relative hydrogen-bond strengths in hydroxybenzoic acids for crystal-engineering purposes [J]. Chem Eur J 19(44):14998–15003
Aakeröy CB, Wijethunga TK, Desper J (2015) Molecular electrostatic potential dependent selectivity of hydrogen bonding [J]. New J Chem 39(2):822–828
Suresh CH, Gadre SR (1998) A novel electrostatic approach to substituent constants: doubly substituted benzenes [J]. J Am Chem Soc 120(28):7049–7055
Gross KC, Seybold PG, Peralta-Inga Z et al (2001) Comparison of quantum chemical parameters and Hammett constants in correlating p K a values of substituted anilines [J]. J Org Chem 66(21):6919–6925
Acknowledgments
The authors thank the 1331 Engineering of Shanxi Province.
Funding
This research was supported by the Basic Research Fund Project (2018JCYJ49) and Educational Reform and Innovation Project (2019JG16) of Modern College of Humanities and Sciences, Shanxi Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Li, X. Intramolecular hydrogen bonding, π-π stacking interactions, and substituent effects of 8-hydroxyquinoline derivative supermolecular structures: a theoretical study. J Mol Model 25, 241 (2019). https://doi.org/10.1007/s00894-019-4140-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4140-2