Abstract
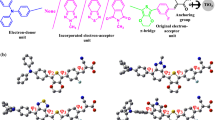
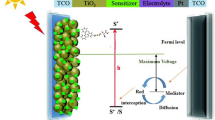
Density functional theory (DFT) and time-dependent DFT (TD-DFT) were used to calculate the properties of the carbazole dyes TYZ-1 to TYZ-5, which differed in their π-spacers. The carbazole dyes TYZ-6 and TYZ-7 were then designed; these were based on TYZ-3 (which had 2,2′:5′,2″-terthiophene as its π-spacer) but had more strongly electron-withdrawing second acceptor groups than TYZ-3. All of these dyes except for TYZ-5 presented quasi-planar conformations, and the calculated energies of their highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) molecular orbitals as well as their HOMO-LUMO gaps (Eg) suggest that these dyes are suitable for use as sensitizers. Lengthening the π-spacer and increasing its degree of conjugation were found to cause the absorption spectrum of the dye to redshift and to facilitate hole injection. The Eg values of TYZ-6 and TYZ-7 were calculated to be smaller than that of TYZ-3 due to the weaker electron-withdrawing power of the second acceptor group in TYZ-3, and the dyes TYZ-2, TYZ-3, TYZ-6, and TYZ-7 presented the smallest Eg values. Local electron excitations following UV-vis absorption led to electronic transitions, particularly HOMO to LUMO transitions (> 94.3% of all transitions). The excited states of these dyes were found to have quasi-planar conformations, although their dihedral angles were smaller than those in the corresponding ground states. The Stokes Shifts calculated for the seven dyes (which ranged from 51.9 to 98.1 nm) suggested that self-absorption was unlikely to occur. Overall, the calculations indicated that the dyes TYZ-2, TYZ-3, TYZ-6, and TYZ-7 are promising candidates for use in dye-sensitized solar cells.






Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Yella A, Lee HW, TSAO HN, Yi CY, Chandiran AK, Nazeeruddin MK, Diau EWG, Yeh CY, Zakeeruddin SM, Grätzel M (2011) Porphyrin-sensitized solar cells with cobalt(II/III)-based electrolyte exceed 12% efficiency. Science 334:629–634
Hagfeldt A, Boschloo G, Sun LC, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Francisco C, Franco J, Abraham A, Padama B (2016) DFT and TD-DFT study on the structural and optoelectronic characteristics of chemically modified donor-acceptor conjugated oligomers for organic polymer solar cells. Polymer 97:55–62
Kavan L, Grätzel M, Gilbert SE, Klemenz C, Scheel HJ (1996) Electrochemical and photoelectrochemical investigation of single-crystal anatase. J Am Chem Soc 118:6716–6723
Kim S, Lee JK, Kang SO, Ko J, Yum JH, Fantacci S, Grätzel M (2006) Molecular engineering of organic sensitizer for solar cell applications. J Am Chem Soc 128:16701–16707
Hagberg DP, Yum JH, Lee H, Angelis FD, Marinado T, Karlsson KM, Nazeeruddin MK (2008) Molecular engineering of organic sensitizer for dye-sensitized solar cell applications. J Am Chem Soc 130:6259–6266
Karuppasamy A, Krishnan KG, Pillai MPV, Ramalingan C (2016) Synthesis, molecular structure and vibrational analysis of D-D-A based carbazole decorated phenothiazine-3-carbaldehyde: experimental (FT-IR, UV and NMR) and density functional theory (DFT) calculations. J Mol Struct 1128:674–684
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BF, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Grätzel M (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242–247
Fu JJ, Duan YA, Zhang JZ, Guo MS, Liao Y (2014) Theoretical investigation of novel phenothiazine-based D-π-A conjugated organic dyes as dye-sensitizer in dye-sensitized solar cells. Comput Theor Chem 1045:145–153
Salvatori P, Amat A, Pastore M, Vitillaro G, Sudhakar K, Giribabu L, Soujanya Y, De Angelis F (2014) Corrole dyes for dye-sensitized solar cells: the crucial role of the dye/semiconductor energy level alignment. Comput Theor Chem 1030:59–66
Srivastava R (2014) On the viability of ruthenium(II) N-heterocyclic carbene complexes as dye-sensitized solar cell (DSSCs): a theoretical study. Comput Theor Chem 1045:47–56
Wu Y, Zhu W, Wu Y (2013) Organic sensitizers from D-π-A to D-A-π-A. Chem Soc Rev 42:2039–2058
Zhang G, Bala H, Cheng Y, Shi D, Lv X, Yu Q, Wang P (2009) High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary π-conjugated spacer. Chem Commun 16:2198–2200
Ren X, Jiang S, Cha M, Zhou G, Wang ZS (2012) Thiophene-bridged double D-π-A dye for efficient dye-sensitized solar cell. Chem Mater 24:3493–3499
Yum JH, Hagberg DP, Moon SJ, Karlsson KM, Marinado T, Sun L, Hagfeldt A, Nazeeruddin MK (2009) A light-resistant organic sensitizer for solar-cell applications. Angew Chem Int Ed 48:1576–1580
Han L, Zhao J, Wang B, Jiang S (2016) Influence of π-bridge in N-fluorenyl indoline sensitizers on the photovoltaic performance of dye-sensitized solar cells. J Photochem Photobiol A Chem 326:1–8
Mishra A, Fischer MKR, Bäuerle P (2009) Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48:2474–2499
Cai SY, Hu XH, Zhang ZY, Su JH, Li X, Ialam A, Han LY, Tian H (2013) Rigid triarylamine-based efficient DSSC sensitizers with high molar extinction coefficients. J Mater Chem A 1:4763–4772
Qu S, Wang B, Guo F, Li J, Wu W, Kong C, Long YT, Hua JL (2012) New diketo-pyrrolo-pyrrole (DPP) sensitizer containing a furan moiety for efficient and stable dye-sensitized solar cells. Dyes Pigments 92:1384–1393
Wang S, Guo J, He L, Wang H, Zhao J, Lu C (2013) Influence of thiophene and benzene unit in triphenylamine dyes on the performance of dye-sensitized solar cells. Synth Met 168:1–8
Murali MG, Wang XZ, Wang Q, Valiyaveettil S (2016) New banana shaped A-D-π-D-A type organic dyes containing two anchoring groups for high performance dye-sensitized solar cells. Dyes Pigments 134:375–381
Wu YZ, Marszalek M, Zakeeruddin SM, Zhang Q, Tian H, Grätzel M, Zhu WH (2012) High-conversion-efficiency organic dye-sensitized solar cells: molecular engineering on D-A-π-A featured organic indoline dyes. Energy Environ Sci 5:8261–8272
Haid S, Marszalek M, Mishra A, Wielopolski M, Teuscher J, Moser JE, Humphry-Baker R, Zakeeruddin SM, Grätzel M, Bäuerle P (2012) Significant improvement of dye-sensitized solar cell performance by small structural modification in π-conjugated donor-acceptor dyes. Adv Funct Mater 22:1291–1302
Cui Y, Wu YZ, Lu XF, Zhang X, Zhou G, Miapeh FB, Zhou LM, Wang ZS (2011) Incorporating benzotriazole moiety to construct D-A-π-A oganic sensitizers for solar sells: significant enhancement of open-circuit photovoltage with long alkyl group. Chem Mater 23:4394–4401
Zhang F, Jiang KJ, Huang JH, Yu CC, Li SG, Chen MG, Yang LM, Song YL (2013) A novel compact DPP dye with enhanced light harvesting and charge transfer properties for highly efficient DSCs. J Mater Chem A 1:4858–4863
Luo GG, Lu H, Wang YH, Dong J, Zhao Y, Wu RB (2016) A D-π-A-π-a metal-free organic dye with improved efficiency for the application of solar energy conversion. Dyes Pigments 134:498–505
Li PP, Chen Y, Zhu J, Feng M, Zhuang X, Lin Y, Zhan H (2011) Charm-bracelet-type poly(N-vinylcarbazole) functionalized with reduced graphene oxide for broadband optical limiting. Chem Eur J 17:780–785
Duan TN, Fan K, Zhong C, Peng TY, Qin JG, Chen XG (2012) New organic dyes containing tert-butyl-capped N-arylcarbazole moiety for dye-sensitized solar cells. RSC Adv 2:7081–7086
Liu J, Yang XD, Islam A, Numata YH, Zhang SF, Salim NT, Chen H, Han LY (2013) Efficient metal-free sensitizers bearing circle chain embracing π-spacers for dye-sensitized solar cells. J Mater Chem A 1:10889–10897
Liu B, Wang R, Mi WJ, Li XY, Yu HT (2012) Novel branched coumarin dyes for dye-sensitized solar cells: significant improvement in photovoltaic performance by simple structure modification. J Mater Chem 22:15379–15387
Zhang Y, Lai SL, Tong QX, Lo MF, Ng TW, Chan MY, Wen ZC, He J, Jeff KS, Tang XL (2011) High efficiency nondoped deep-blue organic light emitting devices based on imidazole-π-triphenylamine derivatives. Chem Mater 24:61–70
Mohr T, Aroulmoji V, Ravindran RS, Müller M, Ranjitha S, Rajarajan G, Anbarasan P (2015) DFT and TD-DFT study on geometries, electronic structures, and electronic absorption of some metal free dye sensitizers for dye sensitized solar cells. Spectrochim Acta A 135:1066–1073
Beck AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372–1377
Beck AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Hertwing RH, Koch W (1997) Excited state properties through cubic response theory: polarizabilities of benzene and naphthalene. Chem Phys Lett 268:345–351
Ku J, Lansac Y, Jang YH (2011) Time-dependent density functional theory study on benzothiadiazole-based low-band-gap fused-ring copolymers for organic solar cell applications. J Phys Chem C 115:21508–21516
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford
Acknowledgements
The authors gratefully acknowledge the funding provided by the National Natural Science Foundation of China (21406202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, YH., Tong, Y., Han, L. et al. Design and photoelectric properties of D-A-π-A carbazole dyes with different π-spacers and acceptors for use in solar cells: a DFT and TD-DFT investigation. J Mol Model 25, 249 (2019). https://doi.org/10.1007/s00894-019-4101-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4101-9