Abstract
A study of the spectroscopic properties of the buckyball dimer (C70)2 was performed, which involved mapping the potential energy curve of this system. The spectroscopic constants of the system were obtained using theoretical Dunham and discrete variable representation methods, as well as the Rydberg analytical function expanded to the sixth degree. Because the fullerenes in the dimer have both hexagonal and pentagonal faces, the properties of (C70)2 were examined for different system configurations. The fullerene dimerization process involves a weak interaction, possibly mediated by short-range components such as van der Waals forces. The differences between the spectroscopic constants of the various (C70)2 configurations and between their dissociation energies De were found to be rather small, which can be attributed to the dominant influence of the hexagonal faces of the fullerenes on the interaction between the fullerenes. These results should aid our understanding of the process of fullerene dimer formation and hopefully facilitate the development and application of new materials based on these dimers.

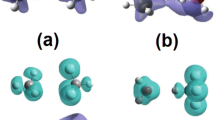
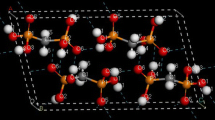
Comparison of the potential energy curve and a schematic representation for the all (C70)2 fullerenes dimers configurations.





Similar content being viewed by others
References
Kroto HW, Heath JR, O’Brien SC et al (1985) C60: buckminsterfullerene. Nature 318:162–163. https://doi.org/10.1038/318162a0
Rodríguez-Fortea A, Balch AL, Poblet JM (2011) Endohedral metallofullerenes: a unique host–guest association. Chem Soc Rev 40:3551. https://doi.org/10.1039/c0cs00225a
Schwerdtfeger P, Wirz LN, Avery J (2015) The topology of fullerenes. Wiley Interdiscip Rev Comput Mol Sci 5:96–145. https://doi.org/10.1002/wcms.1207
Krätschmer W, Lamb LD, Fostiropoulos K, Huffman DR (1990) Solid C60: a new form of carbon. Nature 347:354–358. https://doi.org/10.1038/347354a0
Mojica M, Alonso JA, Méndez F (2013) Synthesis of fullerenes. J Phys Org Chem 26:526–539. https://doi.org/10.1002/poc.3121
Zhang R, Murata M, Aharen T et al (2016) Synthesis of a distinct water dimer inside fullerene C70. Nat Chem 8:435–441. https://doi.org/10.1038/nchem.2464
Nomura K, Okada S (2014) An anomalous dipole–dipole arrangement of water molecules encapsulated into C60 dimer. Chem Phys Lett 608:351–354. https://doi.org/10.1016/j.cplett.2014.06.013
Thompson BC, Fréchet JMJ (2008) Polymer–fullerene composite solar cells. Angew Chem Int Ed 47:58–77. https://doi.org/10.1002/anie.200702506
Thompson BC, Fréchet JMJ (2008) Polymer–Fulleren-Solarzellen. Angew Chem 120:62–82. https://doi.org/10.1002/ange.200702506
Günes S, Neugebauer H, Sariciftci NS (2007) Conjugated polymer-based organic solar cells. Chem Rev 107:1324–1338. https://doi.org/10.1021/cr050149z
Drees M, Hoppe H, Winder C et al (2005) Stabilization of the nanomorphology of polymer–fullerene “bulk heterojunction” blends using a novel polymerizable fullerene derivative. J Mater Chem 15:5158. https://doi.org/10.1039/b505361g
Schroeder BC, Li Z, Brady MA et al (2014) Enhancing fullerene-based solar cell lifetimes by addition of a fullerene dumbbell. Angew Chem Int Ed 53:12870–12875. https://doi.org/10.1002/anie.201407310
Segura JL, Martín N (2000) [60]Fullerene dimers. Chem Soc Rev 29:13–25. https://doi.org/10.1039/a903716k
Menon M, Subbaswamy KR, Sawtarie M (1994) Structure and properties of C60 dimers by generalized tight-binding molecular dynamics. Phys Rev B 49:13966–13969. https://doi.org/10.1103/PhysRevB.49.13966
Fagerström J, Stafström S (1996) Formation of C60 dimers: a theoretical study of electronic structure and optical absorption. Phys Rev B 53:13150–13158. https://doi.org/10.1103/PhysRevB.53.13150
Sabirov DS, Terentyev AO, Bulgakov RG (2014) Polarizability of fullerene [2+2]-dimers: a DFT study. Phys Chem Chem Phys 16:14594. https://doi.org/10.1039/c3cp55528c
Heiney PA, Fischer JE, McGhie AR et al (1991) Heiney et al. reply. Phys Rev Lett 67:1468–1468. https://doi.org/10.1103/PhysRevLett.67.1468
Heiney PA, Fischer JE, McGhie AR et al (1991) Orientational ordering transition in solid C60. Phys Rev Lett 66:2911–2914. https://doi.org/10.1103/PhysRevLett.66.2911
Zettergren H, Rousseau P, Wang Y et al (2013) Formations of dumbbell C118 and C119 inside clusters of C60 molecules by collision with α particles. Phys Rev Lett 110:185501. https://doi.org/10.1103/PhysRevLett.110.185501
Koshino M, Niimi Y, Nakamura E et al (2010) Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomic level. Nat Chem 2:117–124. https://doi.org/10.1038/nchem.482
Wang Y, Zettergren H, Rousseau P et al (2014) Formation dynamics of fullerene dimers C +118 ,C119 +, and C120 +. Phys Rev A 89:38–43. https://doi.org/10.1103/PhysRevA.89.062708
Smith B, Tabin CJ, Monthioux M, Luzzi D (1998) Encapsulated C60 in carbon nanotubes. Nature 396:323–324. https://doi.org/10.1038/24521
Calvaresi M, Bottoni A, Zerbetto F (2015) Thermodynamics of binding between proteins and carbon nanoparticles: the case of C60@lysozyme. J Phys Chem C 119:28077–28082. https://doi.org/10.1021/acs.jpcc.5b09985
Calvaresi M, Zerbetto F, Ciamician CG et al (2010) Baiting proteins with C60. ACS Nano 4:2283–2299. https://doi.org/10.1021/Nn901809b
Tzoupis H, Leonis G, Durdagi S et al (2011) Binding of novel fullerene inhibitors to HIV-1 protease: insight through molecular dynamics and molecular mechanics Poisson–Boltzmann surface area calculations. J Comput Aided Mol Des 25:959–976. https://doi.org/10.1007/s10822-011-9475-4
Yin X, Zhao L, Kang S et al (2013) Impacts of fullerene derivatives on regulating the structure and assembly of collagen molecules. Nanoscale 5:7341. https://doi.org/10.1039/c3nr01469j
Shoji M, Takahashi E, Hatakeyama D et al (2013) Anti-influenza activity of C60 fullerene derivatives. PLoS One 8(6):e66337. https://doi.org/10.1371/journal.pone.0066337
Nierengarten I, Nierengarten JF (2014) Fullerene sugar balls: a new class of biologically active fullerene derivatives. Chem Asian J 9:1436–1444. https://doi.org/10.1002/asia.201400133
Montellano A, Da Ros T, Bianco A, Prato M (2011) Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale 3:4035. https://doi.org/10.1039/c1nr10783f
Machado DFS, Silva VHC, Esteves CS et al (2012) Fully relativistic rovibrational energies and spectroscopic constants of the lowest X: (1)0 + g , A′:(1)2u, A:(1)1u, B’: (1)0 − u and B: (1)0 + u states of molecular chlorine. J Mol Model 18:4343–4348. https://doi.org/10.1007/s00894-012-1429-9
Machado DFS, Silva RAL, de Oliveira AP et al (2017) A novel analytical potential function for dicationic diatomic molecular systems based on deformed exponential function. J Mol Model 23(6):182. https://doi.org/10.1007/s00894-017-3339-3
Soares Neto JJ, Costa LS (1998) Numerical generation of optimized discrete variable representations. Braz J Phys 28:1–11. https://doi.org/10.1590/S0103-97331998000100001
Dunham JL (1932) The energy levels of a rotating vibrator. Phys Rev 41:721–731. https://doi.org/10.1103/PhysRev.41.721
Francl MM, Pietro WJ, Hehre WJ et al (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665. https://doi.org/10.1063/1.444267
Chai J, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615. https://doi.org/10.1039/b810189b
Frisch MJ, Trucks GW, Schlegel HB et al. (2009) Gaussian 09, revision E.01. Gaussian, Inc., Wallingford
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Boys S, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Rydberg R (1933) Uber einige Potentialkurven des Quecksilberhydrids. Z Phys 80:514–524. https://doi.org/10.1007/BF02057312
Mundim KC, Tsallis C (1998) Geometry optimization and conformational analysis through generalized simulated annealing. Int J Quantum Chem 58:373–381. https://doi.org/10.1002/(SICI)1097-461X(1996)58:4<373::AID-QUA6>3.0.CO;2-V
de Andrade MD, Mundim KC, Malbouisson LAC (2008) Convergence of the generalized simulated annealing method with independent parameters for the acceptance probability, visitation distribution, and temperature functions. Int J Quantum Chem 108:2392–2397. https://doi.org/10.1002/qua.21736
da Cunha WF, de Oliveira RM, Roncaratti LF et al (2014) Rovibrational energies and spectroscopic constants for H2O−Ng complexes. J Mol Model 20:2498. https://doi.org/10.1007/s00894-014-2498-8
Rivera Vila HV, Leal LA, Ribeiro LA et al (2012) Spectroscopic properties of the H2+ molecular ion in the 8kπ, 9kσ, 9lπ, 9lσ and 10oσ electronic states. J Mol Spectrosc 273:26–29. https://doi.org/10.1016/j.jms.2012.02.002
Acknowledgements
The authors thank the following Brazilian agencies for financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF), and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG). This research was carried out with the support of the High-Performance Computing Center at the Universidade Estadual de Goiás (UEG). L. Ribeiro and V. H. Carvalho-Silva, in particular, express their gratitude to PROBIP-UEG.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection XIX—Brazilian Symposium of Theoretical Chemistry (SBQT2017)
Electronic supplementary material
ESM 1
(DOCX 1269 kb)
Rights and permissions
About this article
Cite this article
Silva, R.A.L., de Brito, S.F., Machado, D.F.S. et al. The influence of the configuration of the (C70)2 dimer on its rovibrational spectroscopic properties: a theoretical survey. J Mol Model 24, 235 (2018). https://doi.org/10.1007/s00894-018-3780-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3780-y