Abstract
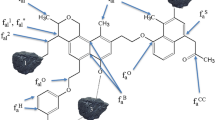
Great progress has been made in the detection of fractions from oxidized lignite, but no molecular structures are reported. A molecular structure model of oxidized Shengli lignite was constructed using ultimate analysis, 13C nuclear magnetic resonance spectrum (NMR), and Fourier transform infrared spectroscopy (FTIR). Parameters are derived from PeakFit4.12 and MestReNova software. Gaussian09 software was used to optimize the model and calculate the FTIR, and the calculated spectrogram is consistent with the experimental one. The molecular formula of the structure model was C96H95O45N, and aromatic rings were mainly linked by oxygen containing functional groups. The electrostatic potential of the structure model was analyzed to explain the great solubility and a good deal of carboxyl acids.






Similar content being viewed by others
References
Miura K (1996) Energy Fuel 10:1196–1201
Miura K, Mae K (1996) Fuel Energy Abstracts 37:425
Mae K (1997) Solubilization of an australian brown coal oxidized with hydrogen peroxide in conventionally used solvents at room temperature. Abstr Pap Am Chem Soc 4:102
Tahmasebi A, Jiang Y, Yu J, Li X, Lucas J (2015) Fuel Process Technol 129:213–221
Mae K, Maki T, Okutsu H, Miura K (2000) Fuel 79:417–425
Karaca H, Ceylan K (1997) Fuel Process Technol 50:19–33
Liu ZX, Liu ZC, Zong ZM, Wei XY, Wang J, Lee CW (2003) Energy Fuel 17:424–426
Liu F, Wei X, Zhu Y, Gui J, Wang Y, Fan X, Zhao Y, Zong Z, Zhao W (2013) Fuel 109:316–324
Liu J, Wei X, Wang Y, Zhang D, Wang T, Lv J, Gui J, Qu M, Zong Z (2015) Fuel 142:268–273
Vlčková Z, Grasset L, Antošová B, Pekař M, Kučerík J (2009) Soil Biol Biochem 41:1894–1901
David J, Šmejkalová D, Hudecová Š, Zmeškal O, Wandruszka RV, Gregor T (2014) Spring 3:156
Mathews JP, Chaffee AL (2012) Fuel 96:1–14
Wender I (1976) Catal Rev 14:97–129
Given PH (1959) Nature 184:980–981
Wiser WH (1984) Conversion of Bituminous Coal to Liquids and Gases: Chemistry and Representative Processes. Springer, Netherlands
Solomon PR (1981) Coal structure and thermal decomposition. ACS Symposium SerIes 169:61–71
Shinn JH (1984) Fuel 63:1187–1196
Feng L, Zhao G, Zhao Y, Zhao M, Tang J (2017) Fuel 203:924–931
Wang J, He Y, Li H, Yu J, Xie W, Wei H (2017) Fuel 203:764–773
Xu F, Pan S, Liu C, Zhao D, Liu H, Wang Q, Liu Y (2017) RSC Adv 7:41512–41519
Meng X, Gao M, Chu R, Miao Z, Wu G, Bai L, Liu P, Yan Y, Zhang P (2017) Chin J Chem Eng 25:1314–1321
Mathews JP, van Duin ACT, Chaffee AL (2011) Fuel Process Technol 92:718–728
Nomura M, Artok L, Murata S, Yamamoto A, Hama H, Gao H, Kidena K (1998) Energy Fuel 12:512–523
Xiao J, Chen S (1998) Chin Sci Bull 43:1048–1050
Machnikowska H, Krztoń A, Machnikowski J (2002) Fuel 81:245–252
Qi X, Wang D, Xin H, Qi G (2014) Spectrosc Lett 47:495–503
Tian B, Qiao YY, Tian YY, Xie KC, Liu Q, Zhou HF (2016) Fuel Process Technol 154:210–218
Li W, Zhu Y, Wang G, Jiang B (2016) Fuel 185:298–304
Qian L, Zhao Y, Sun S, Che H, Chen H, Wang D (2014) Fuel Process Technol 118:327–334
Sönmez Ö, Yıldız Ö, Çakır MÖ, Gözmen B, Giray ES (2018) Fuel 212:12–18
Mani D, Arunan E (2013) Phys Chem Chem Phys 15:14377–14383
Wu J, Liu J, Yuan S, Wang Z, Zhou J, Cen K (2016) Energy Fuel 30:7118–7124
Hagelin H, Murray JS, Politzer P, Brinck T, Berthelot M (1995) Can J Chem 73:483–488
Acknowledgments
The authors are grateful to National Natural Science Foundation of China (Grant No. 51574237, 51274197), and the 111 Project (No.B12030) for the financial support to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Feng, L., Jiang, X. et al. Construction of a molecular structure model of mild-oxidized Chinese lignite using Gaussian09 based on data from FTIR, solid state 13C-NMR. J Mol Model 24, 135 (2018). https://doi.org/10.1007/s00894-018-3677-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3677-9