Abstract
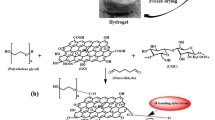
Graphene oxide with different degrees of oxidation was prepared and selected as a model compound of lignite to study quantitatively, using both experiment and theoretical calculation methods, the effect on water-holding capacity of oxygen-containing functional groups. The experimental results showed that graphite can be oxidized, and forms epoxy groups most easily, followed by hydroxyl and carboxyl groups. The prepared graphene oxide forms a membrane-state as a single layer structure, with an irregular surface. The water-holding capacity of lignite increased with the content of oxygen-containing functional groups. The influence on the configuration of water molecule clusters and binding energy of water molecules of different oxygen-containing functional groups was calculated by density functional theory. The calculation results indicated that the configuration of water molecule clusters was totally changed by oxygen-containing functional groups. The order of binding energy produced by oxygen-containing functional groups and water molecules was as follows: carboxyl > edge phenol hydroxyl >epoxy group. Finally, it can be concluded that the potential to form more hydrogen bonds is the key factor influencing the interaction energy between model compounds and water molecules.










Similar content being viewed by others
References
Song Y, Feng W, Li N, Li Y, Zhi K, Teng Y, He R, Zhou H, Liu Q (2016) Effects of demineralization on the structure and combustion properties of Shengli lignite. Fuel 183:659–667
Yang F, Hou Y, Wu W, Niu M (2017) A new insight into the structure of Huolinhe lignite based on the yields of benzene carboxylic acids. Fuel 189:408–418
Feng X, Zhang C, Tan P, Zhang X, Fang Q, Chen G (2016) Experimental study of the physicochemical structure and moisture readsorption characteristics of Zhaotong lignite after hydrothermal and thermal upgrading. Fuel 185:112–121
Schafer HNS (1984) Determination of carboxyl groups in low-rank coals. Fuel 63:723–726
Liu X, Liu S, Fan M, Zhang L (2017) Decrease of hydrophilicity of lignite using CTAB: effects of adsorption differences of surfactant onto mineral composition and functional groups. Fuel 197:474–481
Feng L, Zhao G, Zhao Y, Zhao M, Tang J (2017) Construction of the molecular structure model of the Shengli lignite using TG-GC/MS and FTIR spectrometry data. Fuel 203:924–931
Zhao H, Li Y, Song Q, Wang X, Shu X (2017) Drying, re-adsorption characteristics, and combustion kinetics of Xilingol lignite in different atmospheres. Fuel 210:592–604
Ye C, Huang H, Li X, Li W, Feng J (2017) The oxygen evolution during pyrolysis of HunlunBuir lignite under different heating modes. Fuel 207:85–92
Feng L, Liu X, Song L, Wang X, Zhang Y, Cui T, Tang H (2013) The effect of alkali treatment on some physico–chemical properties of Xilinhaote lignite. Powder Technol 247:19–23
Feng L, Tang J, Ma Z, Wan Y (2016) Effect of mechanical thermal expression drying technology on lignite structure. Dry Technol 35(3):356–362
Yu J, Tahmasebi A, Han Y, Yin F, Li X (2013) A review on water in low rank coals: the existence, interaction with coal structure and effects on coal utilization. Fuel Process Technol 106:9–20
Yu Y, Liu J, Wang R, Zhou J, Cen K (2012) Effect of hydrothermal dewatering on the slurryability of brown coals. Energy Convers Manag 57:8–12
Kelemen SR, Kwiatek PJ (1995) Quantification of organic oxygen species on thesurface of fresh and reacted Argonne premium coal. Energy Fuel 9:841–848
Man C, Liu Y, Zhu X, Che D (2014) Moisture readsorption performance of air-dried and hydrothermally dewatered lignite. Energy Fuel 28:5023–5030
Geng W, Nakajima T, Takanashi H, Ohki A (2009) Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 88:139–144
Tahmasebi A, Yu J, Han Y, Yin F (2012) Study of chemical structure changes of Chinese lignite upon drying in superheated steam, microwave, and hot air. Energy Fuel 26:3651–3660
Norinaga K, Kumagai H, Hayashi J, Chiba T (1998) Classification of water sorbed in coal on the basis of congelation characteristics. Energy Fuel 12:574–579
Wang Y, Wei X, Xie R, Liu F, Li P, Zong Z (2015) Structural characterization of typical organic species in Jincheng no. 15 anthracite. Energy Fuel 29:595–601
Wang Y, Zhou J, Bai L, Chen Y, Zhang S, Lin X (2014) Impacts of inherent O-containing functional groups on the surface properties of Shengli lignite. Energy Fuel 28:862–867
Zhang Y, Jing X, Jing K, Chang L, Bao W (2015) Study on the pore structure and oxygen-containing functional groupsdevoting to the hydrophilic force of dewatered lignite. Appl Surf Sci 324:90–98
Borini S, White R, Wei D (2013) Ultrafast graphene oxide humidity sensors. Nano 12:11166–11173
Chi C, Wang X, Peng Y, Qian Y, Hu Z, Dong J, Zhao D (2016) Facile preparation of graphene oxide membranes for gas separation. Chem Mater 28:2921–2927
Nethravathi C, Rajamathi C, Rajamathi M, Wang X (2014) Cobalt hydroxide/oxide hexagonal Ring_graphene hybrids through chemical etching of metal hydroxide platelets by graphene oxide: energy storage applications. Nano 3:2755–2765
Frost R, Jönsson G, Chakarov D, Svedhem S, Kasemo B (2012) Graphene oxide and lipid membranes: interactions and nanocomposite structures. Nano Lett 12:3356–3362
Wałęsa R, Broda M (2017) The influence of solvent on conformational properties of peptides with Aib residue—a DFT study. J Mol Model 23:349
Arokiyanathan A, Lakshmipathi S (2017) Molecular properties of metal difluorides and their interactions with CO2 and H2O molecules: a DFT investigation. J Mol Model 23:345
Salazar-Villanueva M, Hernandez AB (2017) Adsorption of CO and NO molecules onto pentagonal clusters of aluminum: a DFT study. J Mol Model 23:332
Bourouina A, Rekhis M (2017) Structural and electronic study of iron-based dye sensitizers for solar cells using DFT/TDDFT. J Mol Model 23:310 /1-9
Najafi H, Changizi-Ashtiyani S (2017) Antioxidant activity of omega-3 derivatives and their delivery via nanocages and nanocones: DFT and experimental in vivo investigation. J Mol Model 23:326
Chen X, Wei W, Li L (2017) Catalytic coupling reaction mechanism of 4-nitrobenzenethiol on silver clusters: a density functional theoretical study. J Mol Model 23:321
Roohi H, Ghauri K (2016) Influence of various anions and cations on electrochemical andphysicochemical properties of the nanostructured tunable aryl alkylionic liquids (TAAILs): a DFT M06-2X study. Thermochim Acta 639:20–40
Liu J, Wu J, Zhu J, Wang Z, Zhou J, Cen K (2016) Removal of oxygen functional groups in lignite by hydrothermal dewatering: an experimental and DFT study. Fuel 178:85–92
Wu J, Wang J, Liu J, Yang Y, Cheng J, Wang Z, Zhou J, Cen K (2017) Moisture removal mechanism of low-rank coal by hydrothermal dewatering: physicochemical property analysis and DFT calculation. Fuel 187:242–249
Allardice DJ, Clemow LM, Favas G (2003) The characterisation of different forms of water in low rank coals and some hydrothermally dried products. Fuel 82(6):661–667
Yu J, Tahmasebi A (2013) A review on water in low rank coals: the existence, interaction with coal structure and effects on coal utilization. Fuel Process Technol 106:9–20
Wang G, Yang J, Park J (2008) Facile synthesis and characterization of graphene nanosheets. J Phys Chem C112(22):8192–8195
Liu J, Feng L, Wang X, Zhao M (2017) Exploring the effect of confinement on water clusters in carbon nanotubes. J Mol Model 23:133
Lima I, Sousa L (2017) A DFT study of a set of natural dyes for organic electronics. J Mol Model 23:343
Brahim H, Haddad B (2017) DFT/TDDFT computational study of the structural, electronic and optical properties of rhodium (III) and iridium (III) complexes based on tris-picolinate bidentate ligands. J Mol Model 23:344
Ha N, Cuong N (2017) Electronic properties of the polypyrrole-dopant anions ClO4 − and MoO4 2−: a density functional theory study. J Mol Model 23:336
Michaux C, Wouters J, Perpète EA (2008) Microhydration of protonated glycine: an ab initio family tree. J Phys Chem B 112(8):2430–2438
Bagri A, Mattevi C, Acik M (2010) Structural evolution during the reduction of chemically derived graphene oxide. Nat Chem 2(7):581–587
Charriere D, Behra P (2010) Water sorption on coals. J Colloid Interface Sci 344(2):460–467
Zhang Z (2011) Structure and dynamics in brown coal matrix during moisture removal process by molecular dynamics simulation. Mol Phys 109(3):447–455
D'Arcy R, Watt I (1970) Analysis of sorption isotherms of non-homogeneous sorbents. Trans Faraday Soc 66(569):1236–1245
Mc C, Bandosz T (1999) A molecular model for adsorption of water on activated carbon: comparison of simulation and experiment. Langmuir 15(2):533–544
Domazetis G, James B (2006) Molecular models of brown coal containing inorganic species. Org Geochem 37(2):244–259
Do D, Junpirom S, Do H (2009) A new adsorption-desorption model for water adsorption inactivated carbon. Carbon 47(6):1466–1473
Fei Y, Zhang C (2007) The effect of cationcontent of some raw and ion-exchanged Victorian lignites on their equilibrium moisture content and surface area. Fuel 86(17–18):2890–2897
Norinaga K, Kumagai H (1998) Classification of water sorbed in coal on the basis of congelation characteristics. Energy Fuel 12(3):574–579
Wang X, Feng L (2013) Theoretical study of substituent effects on bond dissociation enthalpies in lignite model compounds. Acta Chim Sin 71(07):10471052
Xiang J, Zeng F (2011) Model construction ofthe macromolecular structure of Yanzhou coal and its molecular simulation. J Fuel Chem Technol 39(7):481–488
Gotch A, Zwier T (1992) Multiphoton ionization studies of clusters of immiscible liquids. I. C6H6–(H2O)n, n=1,2. J Chem Phys 96(5):3388–3401
Pérez J, Hadad C, Restrepo A (2008) Structural studies of the water tetramer. Int J Quantum Chem 108(10):1653–1659
Xu S, Irle S, Musaev DG (2005) Water clusters on graphite: methodology for quantum chemical a priori prediction of reaction rate constants. J Phys Chem A 109(42):9563–9572
Acknowledgments
The authors are grateful to National Natural Science Foundation of China (Grant No. 51574237, 51274197), the National Basic Research Program of China (973 program, Grant No.2012CB214901) and the 111 Project (B12030) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Jiang, X., Cao, Y. et al. Exploring the effect of oxygen-containing functional groups on the water-holding capacity of lignite. J Mol Model 24, 130 (2018). https://doi.org/10.1007/s00894-018-3653-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3653-4