Abstract
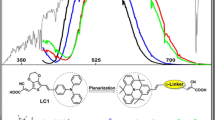
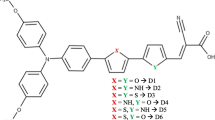
Based on a prototype sensitizer W2, we designed triarylamine-based p-type sensitizers W2-1 to W2-7 that contain modified π-spacers (π'), a π-spacer and two anchors. For W2-1 to W2-4, instead of 2,1,3-benzothiadiazole in W2, thieno[3,4-b]-1,4-dioxin, thiophene, thieno[3,4-c][1,2,5]thiadizole, thiazolo[5,4-d]thiazole are π' and thiophene as π-spacer. For W2-5 to W2-8, π' and π are same, with 2,1,3-benzothiadiazole, thieno[3,4-b]-1,4-dioxin, thieno[3,4-c][1,2,5]thiadiazo, thiazolo[5,4-d]thiazole, respectively, as the π'-spacers. Structure optimization, electronic level and absorption characters were calculated with density functional theory (DFT) and time-dependent DFT (TDDFT) at the CAM-B3LYP/6-311G (d,p). The solvent effect was involved using a polarized continuum model in chloroform. The results showed that the highest occupied molecular orbital and the lowest unoccupied molecular orbital guarantee sufficient hole injection (lower than –0.2 eV), and dye regeneration (lower than –0.2 eV). W2-4 has higher light-harvesting efficiency (LHE) (0.994) and larger overlap with the visible light from 400 nm to 600 nm. Finally, the results suggest that the driving force of hole injection, dye regeneration and charge recombination (ΔGinj, ΔGreg and ΔGCR) of W2-4 are the best, with more negative ΔGinj (–4.33), ΔGreg (–1.74) and more positive ΔGCR (1.92). Replacing 2,1,3-benzothiadiazole with thiazolo[5,4-d]thiazole as π'-spacers is a effective way to improve the performance of the dyes. An introduction of thiazolo[5,4-d]thiazole group can improve the absorption ability and hinder charge recombination.

Absorption spectra of p-type D-π-A sensitizers with modified π-spacers




Similar content being viewed by others
References
Baxter JB, Aydil ES (2005) Appl Phys Lett 86:053114. https://doi.org/10.1063/1.1861510
O'Regan B, Grätzel M (1991) Nature 353(6346):737–740. https://doi.org/10.1038/353737a0
Grätzel M (2001) Nature 414:338–344. https://doi.org/10.1351/pac200173030459
Mathew S, Yella A, Gao P, Humphry Baker R, Curchod BFE, Ashari Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Grätzel M (2014) Nature Chem 6(3):242–247. https://doi.org/10.1038/nchem.1861
Martinez-Diaz MV, de la Torre G, Torres T (2010) Chem Commun 46:7090–7108. https://doi.org/10.1039/C0CC02213F
Imahori H, Umeyama T, Ito S, Large (2009) Acc Chem Res 42:1809. https://doi.org/10.1021/ar900034t
Bessho T, Zakeeruddin SM, Yeh CY (2010) Angew Chem 122:6796. https://doi.org/10.1002/anie.201002118
Mishra A, Fischer MKR, Bäuerle P (2009) Angew Chem Int Ed 48:2474–2499. https://doi.org/10.1002/anie.200804709
Mishra A, Fischer MKR, Bäuerle P (2009) Angew Chem 121:2510–2536. https://doi.org/10.1002/ange.200804709
Ren XF, Kang GJ, He QQ (2016) J Mol Model 22(1):8. https://doi.org/10.1007/s00894-015-2870-3
Ning Z, Tian H (2009) Chem Commun 37:5483. https://doi.org/10.1039/B908802D
Perera IR, Daeneke T, Makuta S, Yu Z, Tachibana Y, Mishra A, Bäuerle P, Ohlin CA, Bach U, Spiccia L (2015) Angew Chem Int Ed 54:3758. https://doi.org/10.1002/anie.201409877
Nattestad A, Mozer AJ, Fischer MKR, Cheng YB, Mishra A, Bauerle P, Bach U (2010) Nat Mater 9:31. https://doi.org/10.1038/nmat2588
Qin P, Zhu H, Edvinsson T, Boschloo G, Hagfeldt A, Sun L (2008) J Am Chem Soc 130(27):8570. https://doi.org/10.1021/ja8001474
Li L, Gibson EA, Qin P, Boschloo G, Gorlov M, Hagfeldt A, Sun L (2010) Adv Mater 22:1759. https://doi.org/10.1002/adma.200903151
Qin P, Linder M, Brinck T, Boschloo G, Hagfeldt A, Sun L (2009) Adv Mater 21:2993–2996. https://doi.org/10.1002/adma.200802461
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) ChemRev 110:6595. https://doi.org/10.1021/cr900356p
Li HB, Zhang J, Wu Y, Jin JL, Duan YA, Su ZM, Geng Y (2014) Dyes Pigments 108:106. https://doi.org/10.1016/j.dyepig.2014.04.029
Huang Z, Natu G, Ji Z, He M, Yu M, Wu Y (2012) J Phys Chem C 116:26239. https://doi.org/10.1021/jp310053f
Wu F, Liu J, Li X, Song Q, Wang M, Zhong C, Zhu L (2015) Eur J Org Chem 31:6850. https://doi.org/10.1002/ejoc.201501036
Liu Z, Li W, Topa S, Xu X, Zeng X, Zhao Z, Wang M, Chen W, Wang F, Cheng YB, He H (2014) ACS Appl Mater Interfaces 6(13):10614. https://doi.org/10.1021/am5022396
Sharma GD, Mikroyannidis JA, Roy MS, Thomas KJ, Ball RJ, Kurchania R (2012) RSC Adv 2(30):11457. https://doi.org/10.1039/C2RA21718J
Dhanabalan A, Van Duren JKJ, Van Hal PA (2001) Adv Funct Mater 11(4):255–262. https://doi.org/10.1002/pola.23582
Schwenn PE, Gui K, Nardes AM, Krueger KB, Lee KH, Mutkins K, Rubinstein-Dunlop H, Shaw PE, Kopidakis N, Burn PL (2011) Adv Energy Mater 1(1):73. https://doi.org/10.1002/aenm.201000024
Odobel F, Pleux L, Pellegrin Y, Blart E (2010) Accounts Chem Res 43(8):1063–1071. https://doi.org/10.1021/ar900275b
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) GAUSSIAN 09 (Revision A.2) Gaussian, Inc., Wallingford
Becke AD (1988) Physical Review A 38(6):3098. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37(2):785. https://doi.org/10.1103/PhysRevB.37.785
Yanai T, Tew D, Handy N (2004) Chem Phys Lett 393(1):51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Iikura H, Tsuneda T, Yanai T, Hirao K (2001) J Chem Phys 115(8):3540–3544. https://doi.org/10.1063/1.1383587
Zhao Y, Truhlar DG (2006) J Phys Chem 110(15):5121–5129. https://doi.org/10.1021/jp060231d
Kang JK, Musgrave CB (2001) J Chem Phys 115(24):11040–11051. https://doi.org/10.1063/1.1415079
Adamo C, Barone V (1999) J Chem Phys 110(13):6158–6170. https://doi.org/10.1063/1.478522
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10(44):6615–6620. https://doi.org/10.1039/B810189B
Qin P, Zhu H, Edvinsson T, Boschloo G, Hagfeldt A, Sun L (2008) J Am Chem Soc 130(27):8570–8571. https://doi.org/10.1021/ja8001474
Zhang F, Yu P, Shen W, Li M, He R (2015) RSC Advances 5(79):64378–64386. https://doi.org/10.1039/C5RA09263A
Acknowledgments
W.Y. thanks the Innovation Project for Postgraduates in Universities of Jiangsu Province and the Innovation Funding from the Graduate School of Nanjing University of Science and Technology (NJUST) (No. SJLX16_0140) for partially supporting this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 94 kb)
Rights and permissions
About this article
Cite this article
Yan, W., Chaitanya, K., Sun, ZD. et al. Theoretical study on p-type D-π-A sensitizers with modified π-spacers for dye-sensitized solar cells. J Mol Model 24, 68 (2018). https://doi.org/10.1007/s00894-018-3596-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3596-9