Abstract
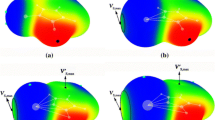
The cation-π interaction can be envisaged as a lewis acid base interaction, and it is in line with Pearson's acid base concept. The critical examination of interactions between the π-acids (alkali metal cations — Li+, Na+ and alkaline earth metal cations Mg2+, Ca2+) on one face and tripodal Cr(CO)3 moiety on the other π face of substituted arenes demonstrates the role of cation and substitutents in manipulating the interactions between them. The interaction of the two π acids on both faces of arene is not expectedly additive, rather it shows either depreciation of interaction energy revealing the competition of acids toward the base or enhancement of interaction energy denoting a cooperative effect. Among the metal cations under study, Mg2+ shows a cooperative gesture. Although the substituents play a meek role, they unfailingly exert their electronic effects and are amply documented by excellent correlation of various parameters with the Hammett constant σm. The elusive switching of λmax from the UV to IR region on binding Mg2+ with substituted arene-Cr(CO)3 complex is a characteristic clue that TDDFT can help design the ionic sensors for Mg2+ cations.


Similar content being viewed by others
References
Meyer EA, Castellano RK, Diederich F (2003) Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed 42:1210–1250. https://doi.org/10.1002/anie.200390319
Salonen LM, Ellermann M, Diederich F (2011) Aromatic rings in chemical and biological recognition: Energetics and structures. Angew Chem Int Ed 50:4808–4842. https://doi.org/10.1002/anie.201007560
Schneider HJ (2009) Binding mechanisms in supramolecular complexes. Angew Chem Int Ed 48:3924–3977. https://doi.org/10.1002/anie.200802947
Dougherty DA (2013) The cation-π interaction. Acc Chem Res 46:885–893. https://doi.org/10.1021/ar300265y
Dougherty DA (1996) Cation-π interactions in chemistry and biology. A new view of benzene, Phe, Tyr, and Trp. Science 271:163–168. https://doi.org/10.1126/science.271.5246
Mecozzi S, West Jr AP, Dougherty DA (1996) Cation-π interactions in simple aromatics: electrostatics provide a predictive tool. J Am Chem Soc 118:2307–2308. https://doi.org/10.1021/ja9539608
Ma JC, Dougherty DA (1997) The cation-π interaction. Chem Rev 97:1303–1324. https://doi.org/10.1021/cr9603744
Mahadevi AS, Sastry GN (2013) Cation-π interaction: its role and relevance in chemistry, biology, and material science. Chem Rev 113:2100–2138. https://doi.org/10.1021/cr300222d
Frontera A, Quinonero D, Deyà PM (2011) Cation-π and anion-π interactions. WIREs Comp Mol Sci 1:440–459. https://doi.org/10.1002/wcms.14
Raju RK, Bloom JWG, An Y, Wheeler SE (2011) Substituents effects on non-covalent interactions with aromatic rings: insights from computational chemistry. ChemPhysChem 12:3116–3130. https://doi.org/10.1002/cphc.201100542
An Y, Wheeler SE (2015) Cation-π interactions. Enc Inorg Bioinorg Chem. doi: https://doi.org/10.1002/9781119951438.eibc2294
Amunugama R, Rodgers MT (2000) Absolute alkali metal ion binding affinities of several azines determined by threshold collision-induced dissociation. Int J Mass Spectrom 195(196):439–457. https://doi.org/10.1016/S1387-3806(99)00145-1
Amunugama R, Rodgers MT (2003) The influence of substituents on cation-π interactions. 5. Binding energies of alkali metal cation-anisole complexes determined by threshold collision-induced dissociation and theoretical studies. Int J Mass Spectrom 222:431–450. https://doi.org/10.1016/S1387-3806(02)00945-4
Amunugama R, Rodgers MT (2002) The influence of substituents on cation-π interactions 1: Binding energies of alkali metal cation-toluene complexes determined by threshold collision-induced dissociation and theoretical studies. J Phy Chem A 106:5529–5539. https://doi.org/10.1021/jp014307b
Indra Neela Y, Sastry GN (2015) Theoretical investigation of anion (F-, Cl-) and cation (Na+) interactions with substituted benzene [C6H6-nYn (Y = –F, –CN, –NO2; n = 1–6)]. Mol Phys Int J Interf Chem Phys 113(2):137–148. https://doi.org/10.1080/00268976.2014
Khanmohammadi A, Raissi H, Mollania F, Hokmabadi L (2014) Molecular structure and bonding character of mono and divalent metal cations (Li+, Na+, K+, Be2+, Mg2+, and Ca2+) with substituted benzene derivatives: AIM, NBO, and NMR analyses. Struct Chem 25:1327–1342. https://doi.org/10.1007/s11224-014-0405-7
Wheeler SE, Houk KN (2009) Substituent effects in cation/π interactions and electrostatic potentials above the centers of substituted benzenes are due primarily to through-space effects of the substituents. J Am Chem Soc 131:3126–3127. https://doi.org/10.1021/ja809097r
Rosillo M, Dominguez G, Perez-Castells J (2007) Chromium arene complexes in organic synthesis. Chem Soc Rev 36:1589–1604. https://doi.org/10.1039/b606665h
Mutterties EL, Bleeke JR, Wuchere EJ, Albright TA (1982) Structural, stereochemical, and electronic features of arene-metal complexes. Chem Rev 82:499–525. https://doi.org/10.1021/cr00051a002
Low AA, Hall MB (2000) Benzene chromium tricarbonyl revisited: theoretical study of the structure and dynamics of (ƞ6-C6H6)Cr(CO)3. Int J Quant Chem 77:152–160. https://doi.org/10.1002/(SICI)1097-461X(2000)77:1<152::AID-QUA14>3.0.CO;2-Q
Suresh CH, Koga N, Gadre SR (2000) Molecular electrostatic potential and electron density topography: structure and reactivity of (substituted arene)Cr(CO)3 complexes. Organometallics 19:3008–3015. https://doi.org/10.1021/om990694o
Feixas F, Jimenez-Halla JOC, Matito E, Poater J, Sola M (2007) Is the aromaticity of the benzene ring in the (ƞ6-C6H6)Cr(CO)3 complex larger than that of the isolated benzene molecule? Polish J Chem 81:783–797
Kalpana A, Akilandeswari L (2015) The effect of fluorine substitution on the conformation and aromaticity of ƞ6-fluoro arene chromium tricarbonl complexes—density functional insights. Comput Theor Chem 1069:125–131. https://doi.org/10.1016/j.comptc.2015.07.020
Kalpana A, Akilandeswari L (2016) Expensive tripodal rotation of ƞ6-chromium tricarbonyl complexes of phosphabenzene-Insights from DFT study. Comput Theor Chem 1084:103–108. https://doi.org/10.1016/j.comptc.2016.03.018
Kalpana A, Akilandeswari L (2017) Tuning the tripodal rotational barrier in ƞ6-chromiumtricarbonyl heteroarenes – A step towards torsional switches. Ind J Chem 56A:610–615
Kalpana A, Akilandeswari L (2017) Structural and electronic effects of cation binding (Li+, Na+, K+, Mg2+ and Ca2+) to π system of ƞ6-benzene-Cr(CO)3 complex: a theoretical study. J Serb Chem Soc. https://doi.org/10.2298/JSC161228063K
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision A.1. Gaussian Inc, Wallingford
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222. https://doi.org/10.1007/BF00533485
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310. https://doi.org/10.1063/1.448975
Boys SF, Bernardi R (1979) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Foster JP, Weinhold F (1980) Natural bond orbitals. J Am Chem Soc 102:7211–7218. https://doi.org/10.1021/ja00544a007
Reed AD, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1988) NBO 3.1 program manual. http://www.ccl.net/cca/software/NT/mopac6/nbo.htm
Schleyer PR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318. https://doi.org/10.1021/ja960582d
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. https://doi.org/10.1021/ja00179a005
Scalmani G, Frisch MJ, Mennucci B, Tomasi J, Cammi R, Barone V (2006) Geometries and properties of excited states in the gas phase and in solution: theory and application of a time-dependent density functional theory polarizable continuum model. J Chem Phy 124:1–15. https://doi.org/10.1063/1.2173258
Skripnikov LV (2009) Chemissian visualization computer program. www.chemissian.com
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comp Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
McDaniel DH, Brown HC (1958) An extended table of Hammett substituent constants based on the ionization of substituted benzoic acids. J Org Chem 23:420–427. https://doi.org/10.1021/jo01097a026
Hansch C, Leo A, Taft RW (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91:165–195. https://doi.org/10.1021/cr00002a004
Yang W, Parr RG (1985) Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci 82:6723–6726
Acknowledgments
The authors thank Prof. P. Venuvanalingam, CSIR Emeritus Professor, School of Chemistry, Bharathidasan University, for his constant support and suggestions. A. Kalpana thanks University Grants Commission, NewDelhi, for the financial assistance (Proposal No:F-MRP-5586/15(SERO/UGC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 514 kb)
Rights and permissions
About this article
Cite this article
Kalpana, A., Akilandeswari, L. Competitive/co-operative interactions in acid base sandwich: role of cation vs. substituents. J Mol Model 23, 341 (2017). https://doi.org/10.1007/s00894-017-3518-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3518-2