Abstract
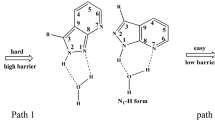
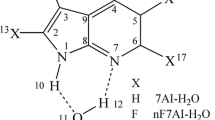
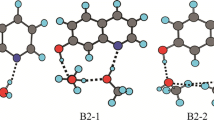
A systematic study on the first excited-state proton transfer (ESPT) in 2RAI-H2O (R = OH, OCH3, CN, NO2, CHO) complexes in solution were investigated at the TD-M06-2X/6-31 + G(d, p) level. The double proton transfer occurred in an asynchronous but concerted protolysis fashion no matter which substituent R was used at C2 position in pyrrole ring in the 7AI-H2O complex. The specific vibrational-mode of ESPT in the model systems was confirmed and contributed to promote the reaction rate by shortening the reaction path. The substituent effects of different groups on the ESPT thermodynamics and kinetics were discussed. It was obvious that the geometries, barrier height, asynchrony, and specific vibration-mode of excited-state proton transfer changed with the different substituent groups.

The distance between two neighboring heavy atoms such as N1-O11 (R1) and O11-N8 (R2) distances played an important role in the proton transfer reaction. The sum of the N1-O11 and O11-N8 distances in the reactant of 2RAI-H2O (R=H, OH, OCH3; CN, CHO, NO2) complexes is in the range of 5.542 Å~5.692 Å and changes along with the substituent group at C2 position in the pyrrole ring. The ESDPT barrier height and the sum of the N1-O11 and O11-N8 distances have a good correlation.







Similar content being viewed by others
Change history
03 November 2017
The original version of this article unfortunately contained a mistake in Table 3. This correct Table 3 is given below.
References
Arnaut LG, Formosinho SJ (1993). J Photochem Photobiol A 75:1
Formosinho SJ, Arnaut LG (1993). J Photochem Photobiol A 75:21
Douhal A, Lahmani F, Zewail AH (1996). Chem. Phys. 207:477
Douhal A, Kim SK, Zewail AH (1995). Nature 378:260
Catalán J, del Valle JC, Kasha M (1999). Proc. Natl. Acad. Sci. 96:8338
Folmer DE, Wisniewski ES, Hurley SM, Castleman Jr SW (1999). Proc. Natl. Acad. Sci. 96:12980
Serrano-Andrés L, Merchán M (2006). Chem. Phys. Lett. 418:569
Takeuchi S, Tahara T (2007). Proc. Natl. Acad. Sci. 104:5285
Kwon OH, Zewail AH (2007). Proc. Natl. Acad. Sci. 104:8703
Sekiya H, Sakota K (2008). J. Photochem. Photobiol. C 9:81
Yu XF, Yamazaki S, Taketsugu T (2011). J. Chem. Theory Comput. 7:1006
Ando K, Hayashi S, Kato S (2011). Phys. Chem. Chem. Phys. 13:11118
Komoto Y, Sakota K, Sekiya H (2005). Chem. Phys. Lett. 406:15
Catalán J, Díaz C, de Paz JLG (2006). Chem. Phys. Lett. 419:164
Hsieh WT, Hsieh CC, Lai CH, Cheng YM, Ho ML, Wang KK, Lee GH, Chou PT (2008). Chem Phys Chem 9:293
Hu WP, Chen JL, Hsieh CC, Chou PT (2010). Chem. Phys. Lett. 485:226
Mukherjee M, Karmakar S, Chakraborty T (2012). Chem. Phys. Lett. 34:519
Yu XF, Yamazaki S, Taketsugu T (2012). J. Comput. Chem. 33:1701
Gordon MS (1996). J. Phys. Chem. 100:3974
Nakajima A, Hirano M, Hasumi R, Kaya K, Watanabe H, Carter C, Williamson J, Miller TA (1997). J. Phys. Chem. A 101:392
Chaban GM, Gordon MS (1999). J. Phys. Chem. A 103:185
Fernández-Ramos A, Smedarchina Z, Siebrand W, Zgierski MZ (2001). J. Chem. Phys. 114:7518
Yokoyama H, Watanabe H, Omi T, Ishiuchi S, Fujii M (2001). J. Phys. Chem. A 105:9366
Casadesús R, Moreno M, Lluch JM (2003). Chem. Phys. 290:319
Kwon OH, Lee YS, Park HJ, Kim YH, Jang DJ (2004). Angew. Chem. Int. Ed. 43:5792
Taketsugu T, Yagi K, Gordon MS (2005). Int. J. Quantum Chem. 104:758
Hara A, Sakota K, Nakagaki M, Sekiya H (2005). Chem. Phys. Lett. 407:30
Sakota K, Komoto Y, Nakagaki M, Ishikawa W, Sekiya H (2007). Chem. Phys. Lett. 435:1
Sakota K, Inoue N, Komoto Y, Sekiya H (2007). J. Phys. Chem. A 111:4596
Kina D, Nakayama A, Noro T, Taketsugu T, Gordon MS (2008). J. Phys. Chem. A 112:9675
Koizumi Y, Jouvet C, Tsuji N, Ishiuchi S, Dedonder-Lardeux C, Fujii M (2008). J. Chem. Phys. 129:104311
Sakota K, Komure N, Ishikawa W, Sekiya H (2009). J. Chem. Phys. 130:224307
Duong MPT, Kim YH (2010). J. Phys. Chem. A 114:3403
Sakota K, Jouvet C, Dedonder C, Fujii M, Sekiya H (2010). J. Phys. Chem. A 114:11161
Fang H, Kim YH (2011). J. Chem. Theory Comput. 7:642
Pino GA, Alata I, Dedonder C, Jouvet C, Sakota K, Sekiya H (2011). Phys. Chem. Chem. Phys. 13:6325
Fang H, Kim YH (2011). J. Phys. Chem. A 115:13743
Daeungern R, Kungwan N, Woschann P, Aquino AJA, Lischka H, Barbatti M (2011). J. Phys. Chem. A 115:14129
Chachisvilis M, Fiebig T, Douhal A, Zewail AH (1998). J. Phys. Chem. A 102:669
Fiebig T, Chachisvilis M, Manger M, Zewial AH, Douhal A, Garciaochoa I, de La Hoz AA (1999). J. Phys. Chem. A 103:7419
Folmer DE, Poth L, Wisniewski ES, Castleman Jr AW (1998). Chem. Phys. Lett. 287:1
Folmer DE, Wisniewski ES, Castleman Jr AW (2000). Chem. Phys. Lett. 318:637
Takeuchi S, Tahara T (1997). Chem. Phys. Lett. 277:340
Takeuchi S, Tahara T (1998). J. Phys. Chem. A 102:7740
Takeuchi S, Tahara T (2001). Chem. Phys. Lett. 347:108
Taylor CA, El-Bayoumi MA, Kasha M (1969). Proc. Natl. Acad. Sci. 63:253
Catalán J, Perez P, del Valle JC, de Paz JLG, Kasha M (2004). Proc. Natl. Acad. Sci. 101:419
Catalán J, de Paz JLG (2005). J. Chem. Phys. 123:114302
Catalán J (2010). J. Phys. Chem. A 114:5666
Sakota K, Hara A, Sekiya H (2004). Phys. Chem. Chem. Phys. 6:32
Sekiya H, Sakota K (2006). Bull. Chem. Soc. Jpn. 79:373
Fang H (2015). Theor. Chem. Acc. 134:142
Hung VP, Robert SP, Audrey GR, Samuel JD, Houk KN (2014). J. Am. Chem. Soc. 136:2397
Chen YL, Wu DY, Tian ZQ (2016). J. Phys. Chem. A 120:4049
Chou PT, Yu WS, Wei CY, Cheng YM, Yang CY (2001). J. Am. Chem. Soc. 123:3599
Tseng MY, Hung HY, Sung KS (2015). J. Phys. Chem. A 119:3905
Poenitzsch VZ, Winters DC, Xie H, Dieckmann GR, Dalton AB, Musselman IH (2007). J. Am. Chem. Soc. 129:14724
Tseng HW, Liu JQ, Chen YA, Chao CM, Liu KM, Chen CL, Lin TC, Hung CH, Chou YL, Lin TC, Wang TL, Chou PT (2015). J. Phys. Chem. Lett. 6:1477
Chen YA, Meng FY, Hsu YH, Hung CH, Chen CL, Chung KY, Tang WF, Hung WY, Chou PT (2016). Chem. Eur. J. 22:14688
Zhao Y, Truhlar DG (2008). Theor. Chem. Accounts 120:215
Frisch MJ, Truck GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford,
Cancès E, Mennucci B, Tomasi J (1997). J. Chem. Phys. 107:3032
Cossi M, Barone V, Mennucci B, Tomasi J (1998). Chem. Phys. Lett. 286:253
Mennucci B, Tomasi J (1997). J. Chem. Phys. 106:5151
Glendening ED (2005). J. Phys. Chem. A 109:11936
Glendening ED, Landis CR, Weinhold F (2013). J. Comput. Chem. 35:1429
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge, University Press, Cambridge,
Mohammed OF, Pines D, Nibbering ETJ, Pines E (2006). Angew. Chem. Int. Ed. 46:1458
Yi J, Fang H (2017). Photochem. Photobiol. https://doi.org/10.1111/php.12839
Hansch C, Leo A, Taft RW (1991). Chem. Rev. 91:165
Brown ID (1992). Acta Cryst B 48:553
Limbach HH, Pietrzak M, Benedict H, Tolstoy PM, Golubev NS, Denisov GS (2004). J. Mol. Struct. 706:115
Limbach HH, Lopez JM, Kohen A (2006). Philos. Trans. R. Soc. B 361:1399
Limbach HH (2007) In: Schowen RL, Klinman JP, Hynes JT, Limbach HH (eds) In hydrogen-transfer reactions. Wiley, Weinheim, Chapter 6, p. 135221
Fang WH (1998). J. Am. Chem. Soc. 120:7568
Fang WH (1999). J. Am. Chem. Soc. 103:5567
Limbach HH, Schowen KB, Schowen RL (2010). J. Phys. Org. Chem. 23:586
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (21403114), the Natural Science Foundation of Jiangsu province (BK20140970), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, state Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s00894-017-3506-6.
Rights and permissions
About this article
Cite this article
Yi, J., Fang, H. Theoretical investigation on the water-assisted excited-state proton transfer of 7-azaindole derivatives: substituent effect. J Mol Model 23, 312 (2017). https://doi.org/10.1007/s00894-017-3487-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3487-5