Abstract
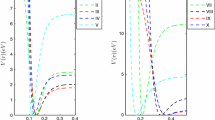
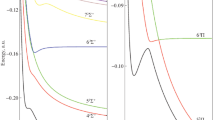
In this paper, we propose a new alternative analytical function aiming to better describe the potential energy curves of the doubly charged diatomic molecules. To achieve this goal, we modified an existing potential function in the literature to describe dicationic diatomic molecules using the deformed exponential function. We generated the potential energy curve of the testing group of dicationic diatomic molecules \( \mathrm{B}{\mathrm{e}}_2^{2+} \), BH2+, \( \mathrm{H}{\mathrm{e}}_2^{2+} \) and NH2+ by means of the CCSD(T)/aug-cc-pVQZ level of theory. To validate this new function, we also calculated the spectroscopic constants and the rovibrational spectra for the electronic state \( {X}^1{\varSigma}_g^{+} \)of the \( \mathrm{B}{\mathrm{e}}_2^{2+} \) and \( \mathrm{H}{\mathrm{e}}_2^{2+} \) systems using the Dunham and discrete variable representation methods. For BH2+ and NH2+ molecules, despite exhibiting a local minimum in the potential energy curve, no vibrational levels are supported, so the spectroscopic constants for these poorly bound systems are invalidated. The fitting accuracy had a better performance over the original potential for describing dicationic diatomic systems, considering that the discrete variable representation method resulted in a similar vibrational structure described in the literature. This fact can be explained due to the deformed function’s flexibility.




Similar content being viewed by others
References
Mathur D (2004) Structure and dynamics of molecules in high charge states. Phys Rep 391:1–118. doi:10.1016/j.physrep.2003.10.016
Price SD (2007) Coincidence studies of the bond-forming reactivity and reaction dynamics of molecular dications. Int J Mass Spectrom 260:1–19. doi:10.1016/j.ijms.2006.06.018
Rodríguez-Mercado JJ, Mateos-Nava RA, Altamirano-Lozano MA (2011) DNA damage induction in human cells exposed to vanadium oxides in vitro. Toxicol Vitr 25:1996–2002. doi:10.1016/j.tiv.2011.07.009
Schröder D, Schwarz H (1999) Generation, stability, and reactivity of small, multiply charged ions in the gas phase. J Phys Chem A 103:7385–7394. doi:10.1073/pnas.1004728107
Shepperson B, Liu J, Ellis AM, Yang S (2011) Ionization of doped helium nanodroplets: residual helium attached to diatomic cations and their clusters. J Phys Chem A 115:7010–7016. doi:10.1021/jp112204e
De Oliveira-Filho AGS, Ornellas FR (2013) The surprising metastability of TeH2+. J Chem Phys doi:10.1063/1.4809566
Alves TV, Hermoso W, Franzreb K, Ornellas FR (2011) Calcium-containing diatomic dications in the gas phase. Phys Chem Chem Phys 13:18297. doi:10.1039/c1cp20735k
Linguerri R, Hochlaf M, Bacchus-Montabonel M-C, Desouter-Lecomte M (2013) Characterization of the MgO2+ dication in the gas phase: electronic states, spectroscopy and atmospheric implications. Phys Chem Chem Phys 15:824–831. doi:10.1039/c2cp43576d
Esteves CS, de Oliveira HCB, Ribeiro L, et al (2006) Modeling diatomic potential energy curves through the generalized exponential function. Chem Phys Lett 427:10–13. doi:10.1016/j.cplett.2006.06.020
Nenajdenko VG, Shevchenko NE, Balenkova ES, Alabugin IV (2003) 1,2-Dications in organic main group systems. Chem Rev 103:229–282. doi:10.1021/cr0000628
Babb JF, Du ML (1990) Quantum-mechanical calculation of quasi-bound energies and resonance widths for the helium molecular dication He22+. Chem Phys Lett 167:273–277. doi:10.1016/0009-2614(90)87167-P
Nicolaides CA (1989) Energy generation from volcanic ground states. Application to cold He22+. Chem Phys Lett 161:547–553. doi:10.1016/0009-2614(89)87036-8
Salviano LR, Esteves CS, de Oliveira HCB, et al (2010) Use of generalized exponential function to build three-dimensional reactive surfaces. Phys A Stat Mech its Appl 389:3604–3612. doi:10.1016/j.physa.2010.04.031
de Oliveira HCB, Rangel FC, Esteves CS et al. (2009) Calculation of MP2 and coupled-cluster molecular properties using the q-integral method. J Phys Chem A 113:14691–14698. doi:10.1021/jp904807b
Wang F, Yang C, Zhu Z (2004) New analytical potential energy function for diatomic cations. J Mol Struct THEOCHEM 684:9–13. doi:10.1016/j.theochem.2004.06.002
Zhang Y-G, Zha X-W, Gao T (2012) Theoretical study on the low-lying electronic states of he 2, he + 2, and he ++ 2. Commun Theor Phys 57:1048–1052. doi:10.1088/0253-6102/57/6/18
Royappa AT, Suri V, McDonough JR (2006) Comparison of empirical closed-form functions for fitting diatomic interaction potentials of ground state first- and second-row diatomics. J Mol Struct 787:209–215. doi:10.1016/j.molstruc.2005.11.008
Sabzyan H, Keshavarz E, Noorisafa Z (2014) Diatomic dications and dianions. J Iran Chem Soc 11:871–945. doi:10.1007/s13738-013-0359-5
Dunham JL (1932) The energy levels of a rotating vibrator. Phys Rev 41:721–731
Soares Neto JJ, Costa LS (1998) Numerical generation of optimized discrete variable representations. Brazilian J Phys 28:1–11. doi:10.1590/S0103-97331998000100001
Light JC, Hamilton IP, Lill JV (1985) Generalized discrete variable approximation in quantum mechanicsa. J Chem Phys 82:1400. doi:10.1063/1.448462
Machado DS, Silva VC, Esteves C et al (2012) Fully relativistic rovibrational energies and spectroscopic constants of the lowest X:(1)0(+)g, A’:(1)2( u ), A:(1)1 ( u ), B’:(1)0(-)u and B:(1)0(+)u states of molecular chlorine. J Mol Model 18:4343–4348. doi:10.1007/s00894-012-1429-9
da Fonsêca JE, de Oliveira HCB, da Cunha WF, Gargano R (2014) Alternative analytical forms to model diatomic systems based on the deformed exponential function. J Mol Model 20:2297. doi:10.1007/s00894-014-2297-2
Rangel FC, de Oliveira HCB, Montel ALB, Mundim KC (2010) Calculation of DFT molecular properties using the integral method. Phys A Stat Mech its Appl 389:5208–5215. doi:10.1016/j.physa.2010.06.030
Amador DHT, de Oliveira HCB, Sambrano JR, Gargano R, de Macedo LGM (2016) 4-Component correlated all-electron study on Ekaactinium Fluoride (E121F) including Gaunt interaction: accurate analytical form, bonding and influence on rovibrational spectra. Chem Phys Lett 662:169–175
Coutinho ND, Silva VHC, Mundim KC, de Oliveira HCB (2015) Description of the effect of temperature on food systems using the deformed Arrhenius rate law: deviations from linearity in logarithmic plots vs. inverse temperature. Rend Lincei 26:141–149. doi:10.1007/s12210-015-0407-4
Carvalho-Silva VH, Aquilanti V, de Oliveira HCB, Mundim KC (2017) Deformed transition-state theory: deviation from Arrhenius behavior and application to bimolecular hydrogen transfer reaction rates in the tunneling regime. J Comput Chem 38:178–188. doi:10.1002/jcc.24529
Santin LG, Toledo EM, Carvalho-Silva VH et al. (2016) Methanol solvation effect on the proton rearrangement of curcumin’s enol forms: an ab initio molecular dynamics and electronic structure viewpoint. J Phys Chem C 120(36):19923–19931
Claudino D, Gargano R, Carvalho-Silva VH, et al (2016) Investigation of the abstraction and dissociation mechanism in the nitrogen trifluoride channels: combined post-Hartree-Fock and transition state theory approaches. J Phys Chem A 120:5464–5473. doi:10.1021/acs.jpca.6b04947
Mundim KC, Tsallis C (1996) Geometry optimization and conformational analysis through generalized simulated annealing. Int J Quantum Chem 58:373–381
de Andrade MD, Mundim KC, Malbouisson LAC (2005) GSA algorithm applied to electronic structure: Hartree-Fock-GSA method. Int J Quantum Chem 103:493–499. doi:10.1002/qua.20580
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308–313
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–441
Amador DHT, de Oliveira HCB, Sambrano JR, et al (2016) 4-component correlated all-electron study on Eka-actinium fluoride (E121F) including gaunt interaction: accurate analytical form, bonding and influence on rovibrational spectra. Chem Phys Lett 662:169–175. doi:10.1016/j.cplett.2016.09.025
Pople JA, Head-Gordon M (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975. doi:10.1063/1.453520
Woon T, Dunning Jr TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371. doi:10.1063/1.464303
Frisch MJ, Trucks GW, Schlegel HB et al. (2009) Gaussian 09, revision D.01. Gaussian Inc, Wallingford
Wang FH, Zhu ZH, Jing FQ (1998) Analytic potential energy function for doubly charged ions BH2+, CH2+ and NH2+. J Mol Struct THEOCHEM 453:71–75. doi:10.1016/S0166-1280(98)00181-X
Nicolaides CA, Chrysos M, Valtazanos P (1990) Stability and physicochemical reactions of light dications. J Phys B Atomic Mol Phys 23:791–800. doi:10.1088/0953-4075/23/5/004
Olsen J, Jørgensen P, Helgaker T, Christiansen O (2000) Divergence in Moller-Plesset theory: a simple explanation based on a two-state model. J Chem Phys 112:9736–9748
Acknowledgements
The authors thank the following Brazilian agencies for financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF) and Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG). L. Ribeiro and V. H. Carvalho-Silva, in particular, express their gratitude to PROBIP-UEG.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper belongs to Topical Collection VI Symposium on Electronic Structure and Molecular Dynamics – VI SeedMol
Electronic supplementary material
ESM 1
(DOCX 121 kb)
Rights and permissions
About this article
Cite this article
Machado, D.F.S., Silva, R.A.L., de Oliveira, A.P. et al. A novel analytical potential function for dicationic diatomic molecular systems based on deformed exponential function. J Mol Model 23, 182 (2017). https://doi.org/10.1007/s00894-017-3339-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3339-3