Abstract
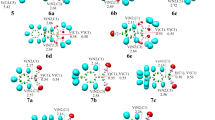
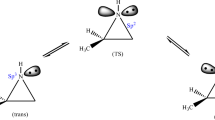
A series of theoretical computations were conducted via density functional theory at the B3LYP/6–31 + G(d,p) level to study the mechanism of the organocatalytic synthesis of a benzoxazine-substituted indolizine derivative. Four possible alternative pathways were considered in this work. The calculated results show that the formation of an N-ylide precursor from 4-dimethylaminopyridine (DMAP) is a key step as it provides the necessary nucleophilic centre for the subsequent H-migration and H-elimination processes. The precursor N-ylide and Schiff base isomers with the most favourable activities in the preliminary work were identified theoretically by analysing the reaction mechanism. The synthetic mechanism to obtain the indolizine derivative was found to be a two-step reaction, with the rate-determining step being the first H migration to form a transition state with a four-membered ring. The catalytic activity of DMAP in the first H-migration step in the overall synthetic process greatly reduces the reaction barrier height. The chiral selectivity of the synthesis is dominated by the spatial geometry of the Schiff base functional group.















Similar content being viewed by others
Change history
30 December 2019
The original version of this article unfortunately contained a mistake. The presentation of Diagram 2, Fig. 2 and Fig. 3 were incorrect.
References
Debache A, Ghalem W, Boulcina R, Belfaitah A, Rhouati S, Carboni B (2009) Tetrahedron Lett 50:5248–5250
Debache A, Boulcina R, Belfaitah A, Rhouati S, Carboni B (2008) Synlett 4:509–512
Debache A, Ghalem W, Boulcina R, Belfaitah A, Rhouati S, Carboni B (2010) Lett Org Chem 7:272–276
Ramachary DB, Mondal R, Jain S (2016) ARKIVOC 2:98–115
Lee J, Muthaiah S, Hong SH (2014) Adv Synth Catal 356:2653–2660
Derabli C, Boulcina R, Kirsch G, Carboni B, Debache A (2014) Tetrahedron Lett 55:200–204
Shi Q, Tan ZC, Di YY, Tong B, Li YS, Wang SX (2017) J Chem Eng Data 52:941–947
Murugan R, Scriven EF (2003) Aldrichim Acta 31:21–27
Christoph G (2003) Synlett 10:1568–1569
Ragnarsson U, Grehn L (1998) Acc Chem Res 31:494–501
Wang Y, Kataeva O, Metz P (2009) Adv Synth Catal 351:2075–2080
Bappert E, Müller P, Fu GC (2006) Chem Commun 24:2604–2606
Chaudhary S, Hernandez O (1979) Tetrahedron Lett 20:99–102
Xie J, Sha F, Wu XY (2016) Tetrahedron 72:4047–4054
Zhao GL, Huang JW, Shi M (2003) Org Lett 5:4737–4739
Sakakura A, Kawajiri K, Ohkubo T, Kosugi Y, Ishihara K (2007) J Am Chem Soc 129:14775–14779
Shang YJ, Wang CE, He XW, Ju K, Zhang M, Yu SY, Wu JP (2010) Tetrahedron 66:9629–9633
Khan AT, Lal M, Ali S, Khan MM (2011) Tetrahedron Lett 52:5327–5332
Busto E, Gotor-Fernández V, Gotor V (2006) Tetrahedron Asymmetry 17:1007–1016
Sun XX, Zhang HH, Li GH, Meng L, Shi F (2016) Chem Commun 52:2968–2971
Haimov E, Nairoukh Z, Shterenberg A, Berkovitz T, Jamison TF, Marek I (2016) Angew Chem Int Ed 55:5517–5520
Adhikari D, Nguyen ST, Baik MH (2014) Chem Commun 50:2676–2678
Roshan KR, Palissery RA, Kathalikkattil AC, Babu R, Mathai G, Lee HS, Park DW (2016) Catal Sci Technol 6:3997–4004
Xu SJ, Held I, Kempf B, Mayr H, Steglich W, Zipse H (2005) Chem Eur J 11:4751–4757
Smith SC, Clarke ED, Ridley SM, Bartlett D, Greenhow DT, Glithro H, Klong AY, Mitchell G, Mullier GW (2005) Pest Manag Sci 61:16–24
Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M (1996) J Med Chem 39:3636–3658
Marcos M, Serrano JL, Sierra T, Gimenez MJ (1993) Chem Mater 5:1332–1337
Asahina Y, Takei M, Kimura T, Fukuda Y (2008) J Med Chem 51:3238–3249
Bourlot AS, Sanchez I, Dureng G, Guillaumet G, Massingham R, Monteil A, Winslow E, Pujol MD, Merour JY (1998) J Med Chem 41:3142–3158
Phillips OA, Sharaf LH (2016) Expert Opin Ther Pat 26:591–605
Wakabayashi H, Narita T, Suga A (2010) In Vivo 24:39–44
Mousset D, Rabot R, Bouyssou P, Coudert G, Gillaizeau I (2010) Tetrahedron Lett 51:3987–3990
De Bolle L, Andrei G, Snoeck R, Zhang Y, Van Lommel A, Otto M, Bousseau A, Roy C, De Clercq E, Naesens L (2004) Biochem Pharmacol 67:325–336
Blattes E, Lockhart B, Lestage P, Schwendimann L, Gressens P, Fleury MB, Largeron M (2005) J Med Chem 48:1282–1286
Ilas J, Jakopin Z, Borstnar T, Stegnar M, Kikelj D (2008) J Med Chem 51:5617–5629
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al (2009) Gaussian 09, revision a.02. Gaussian, Inc., Wallingford
Acknowledgements
The authors thank the National Natural Science Foundation of China (21373012) and the generous support provided by the Supercomputing Center of the University of Science and Technology of China in the form of computing time.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
The tables list all key bond lengths for the structures involved in the reaction processes, and the Z-matrices for the corresponding optimized structures obtained from the DFT calculations. (DOC 770 kb)
Rights and permissions
About this article
Cite this article
Mao, X., Wang, S. & Shang, Y. A DFT study on the mechanism of the organocatalytic synthesis of a benzoxazine-substituted indolizine derivative. J Mol Model 23, 177 (2017). https://doi.org/10.1007/s00894-017-3328-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3328-6