Abstract
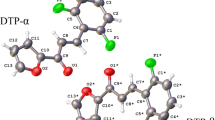
Chalcones are an important class of natural compounds that exhibit numerous biological activities. In this paper, we report the synthesis and characterization of new fluorinated chalcone (FCH). The molecular geometry was determined by means of single crystal X-ray diffraction, and density functional theory (DFT) at B3LYP, M06-2X functionals and MP2 method, with the 6-311++G(d,p) basis set, was applied to optimize the ground state geometry and to study the molecular conformational stability. The molecular electrostatic potential (MEP) was also investigated at the same level of theory in order to identify and quantify the possible reactive sites. The FCH crystallizes in the centrossymmetric space group \( P\overline{1} \) with two independent conformers (α and β) in the asymmetric unit cell. The α conformer is arranged in planar layer whereas the β creates a layer of non-classical dimer along c axis, that differ from α in about 11° in the orientation of phenyl groups. The stabilization of the β conformer is achieved by C−H···π arrangement. The small energy difference between the conformers (0.086 kcal mol−1) and the absence of activation energy indicates that the conversion between them can takes place at room temperature and the β isomer is stable only in solid state. The FCH most electrophilic site occurs on the oxygen atom from the carboxyl group with absolute MEP value of about −52 kcal mol−1 whereas the MEP value calculated for F site is about −23 kcal mol−1.









Similar content being viewed by others
References
Newman DJ, Cragg GM (2012) Natural products as sources of New drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125–137. doi:10.1016/j.ejmech.2006.09.019
Lamim M, Domeneghini L, Rosaria L, Dias S, Kramer L, Regina T et al. (2011) Bioorganic & medicinal chemistry trimethoxy-chalcone derivatives inhibit growth of leishmania braziliensis: synthesis, biological evaluation, molecular modeling and structure–activity relationship (SAR). Bioorg Med Chem Elsevier Ltd 19:5046–5052. doi:10.1016/j.bmc.2011.06.023
Nielsen SF, Christensen SB, Cruciani G, Kharazmi A, Liljefors T (1998) Antileishmanial chalcones: statistical design, synthesis, and three-dimensional quantitative structure–activity relationship analysis. J Med Chem 41:4819–4832
Zhai L, Chen M, Blom J, Theander TG, Kharazmi A (1999) The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother 43:793–803
Lin Y, Zhou Y, Flavin MT, Zhou L, Nie W, Chen F (2002) Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem 10:2795–2802
Lahtchev KL, Batovska DI, Parushev SP, Ubiyvovk VM, Sibirny AA (2008) Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur J Med Chem 43:2220–2228. doi:10.1016/j.ejmech.2007.12.027
Sivakumar PM (2009) Antifungal activity, mechanism and QSAR studies on chalcones. Chem Biol Drug Des 74:68–79. doi:10.1111/j.1747-0285.2009.00828.x
Padhye S, Ahmad A, Oswal N, Dandawate P, Rub RA, Deshpande J et al. (2010) Bioorganic & medicinal chemistry letters fluorinated 2 0 -hydroxychalcones as garcinol analogs with enhanced antioxidant and anticancer activities. Bioorg Med Chem Lett Elsevier Ltd 20:5818–5821. doi:10.1016/j.bmcl.2010.07.128
Kumar PCR, Ravindrachary V, Janardhana K, Manjunath HR, Karegouda P, Crasta V et al. (2011) Optical and structural properties of chalcone NLO single crystals. J Mol Struct Elsevier BV 1005:1–7. doi:10.1016/j.molstruc.2011.07.038
Ghouili A, Dusek M, Petricek V, Ben AT, Ben HR (2013) Synthesis, crystal structure and spectral char- acteristics of highly fluorescent chalcone- based coumarin in solution and in polymer matrix. J Phys Chem Solids Elsevier. doi:10.1016/j.jpcs.2013.09.011
D’silva ED, Podagatlapalli GK, Rao SV, Rao DN, Dharmaprakash SM (2011) New, high efficiency nonlinear optical chalcone co-crystal and structure–property relationship. Cryst Growth Des 11:5362–5369. doi:10.1021/cg2009539
Bukhari SN, Jasamai M, Jantan I (2012) Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev Med Chem;12. doi:10.2174/13895575112091394
Suwunwong T, Chantrapromma S, Fun H (2011) Influence of trimethoxy-substituted positions on fluorescence of heteroaryl chalcone derivatives. Versita 65:890–897. doi:10.2478/s11696-011-0084-4
Prasad YR, Rao AL, Rambabu R (2008) Synthesis and antimicrobial activity of some chalcone derivatives. E-J Chem 5:461–466
Nakamura C, Kawasaki N, Miyataka H, Jayachandran E, Kim H, Kirk KL et al. (2002) Synthesis and biological activities of fluorinated chalcone derivatives. Bioorg Med Chem 10:699–706
Banks RE, Smart BE, Tatlow JC (1994) Organofluorine chemistry: principles and commercial applications. Springer, Heidelberg
Steed JW (2003) Should solid-state molecular packing have to obey the rules of crystallographic symmetry? CrystEngComm 32:169–179. doi:10.1039/b304631a
Bishop R, Scudder ML (2009) Multiple molecules in the asymmetric unit (Z′ > 1) and the formation of false conglomerate crystal structures. Cryst Growth Des 9:2890–2894. doi:10.1039/B900463G
Desiraju GR (2007) On the presence of multiple molecules in the crystal asymmetric unit. CrystEngComm 9:91–92. doi:10.1039/b614933b
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4:378–392. doi:10.1039/b203191b
McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr Sect B: Struct Sci. doi:10.1107/S0108768104020300
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis; 19–32. doi:10.1039/b818330a
McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA, Grimwood DJ, Wolff SK (2012) Crystal Explorer (version 3.1). University of Western Australia
Groom CR, Allen FH (2014) The Cambridge Structural Database in retrospect and prospect. Angew Chem Int Ed 53:662–671. doi:10.1002/anie.201306438
Battle GM, Ferrence GM, Allen FH (2010) Applications of the Cambridge Structural Database in chemical education. J Appl Crystallogr Int Union Crystallogr 43:1208–1223. doi:10.1107/S0021889810024155
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. doi:10.1103/PhysRev.140.A1133
Hohenbergt P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871. doi:10.1103/PhysRev.136.B864
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al. (2009) Gaussian 09, revision A.02. Gaussian Inc, Wallingford. doi:10.1159/000348293
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648. doi:10.1063/1.464913
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other fun. Theor Chem Accounts 120:215–241. doi:10.1007/s00214-007-0310-x
Ternavisk RR, Camargo AJ, Machado FBC, Rocco JAFF, Aquino GLB, Silva VHC et al. (2014) Synthesis, characterization, and computational study of a new dimethoxy-chalcone. J Mol Model 20:2526. doi:10.1007/s00894-014-2526-8
Head-Gordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Chem Phys Lett Accounts 153:503–506
Soltani S, Haghaei H, Shayanfar A, Vallipour J, Asadpour Zeynali K, Jouyban A et al. (2013) Exploring chemistry with electronic structure methods. p. 335. doi:10.1002/adma.200400767
Galabov B, Nikolova V, Ilieva S (2013) Does the molecular electrostatic potential reflect the effects of substituents in aromatic systems? Chem Eur J 19:5149–5155. doi:10.1002/chem.201204092
Fun H-K, Jebas SR, Patil PS, Dharmaprakashc SM (2008) (E)-1-(4-Chlorophenyl)-3-(4-methylphenyl)prop-2-en-1-one. Acta Crystallogr Sect E Struct Rep Online 64:1038
Fun H-K, Patil PS, Dharmaprakash SM, Suchada C (2008) 1-(4-Bromophenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one. Acta Crystallogr Sect E Struct Rep Online 64:1540–1541
De Castro MRC, Araga AQ, Hamilton B (2013) An additional methylene group driving the conformation and assembly of two arylsulfonamide para-alkoxychalcone hybrids. Acta Crystallogr Sect C 69:267–272. doi:10.1107/S0108270113002291
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem Int Union Crystallogr 71:3–8. doi:10.1107/S2053229614024218
Aitipamula S, Desiraju GR, Jasko M, Nangia A, Thaimattam R (2003) Multiple molecules in the crystallographic asymmetric unit. Self host–guest and doubly interpenetrated hydrogen bond networks in a pair of keto-bisphenols. CrystEngComm 78:447–450. doi:10.1039/b312085f
Martinez CR, Iverson BL (2012) Rethinking the term “pi-stacking.”. Chem Sci 3:2191. doi:10.1039/c2sc20045g
Janiak C (2000) A critical account on–stacking in metal complexes with aromatic nitrogen-containing ligands. J Chem Soc, Dalt Trans;3885–3896. doi:10.1039/b003010o
Alvarez S (2013) Dalton Transactions. Dalton Trans. doi:10.1039/c3dt50599e
Vaz WF, Custodio JMF, Silveira RG, Castro AN, Campos CEM, Anjos MM et al. (2016) Synthesis, characterization, and third-order nonlinear optical properties of a new neolignane analogue. RSC Adv 6:79215–79227. doi:10.1039/C6RA14961H
Boys SF, Bernardi F (2006) The calculation of small molecular interactions by the differences of separate total energies . Some procedures with reduced errors. Mol Phys; 37–41.
Salvador P, Simon S, Duran M, Dannenberg JJ, Salvador P, Simon S et al. (2013) C–HO H-bonded complexes: How does basis set superposition error change their potential-energy surfaces doi:10.1063/1.1290010
Dhar DN (1981) The chemistry of chalcones and related compounds. Wiley, New York
Enraf-Nonius (1993) CAD-4/PC Software, version 1.2; Enraf-Nonius: Delft, The Netherlands
Acknowledgements
The authors would like to acknowledge the National Council of Technological and Scientific Development (CNPq, Brazil) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection VI Symposium on Electronic Structure and Molecular Dynamics – VI SeedMol
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 38 kb)
Rights and permissions
About this article
Cite this article
Carvalho, P.S., Custodio, J.M.F., Vaz, W.F. et al. Conformation analysis of a novel fluorinated chalcone. J Mol Model 23, 97 (2017). https://doi.org/10.1007/s00894-017-3245-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3245-8