Abstract
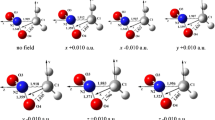
The effects of an external electric field on the C/N–NO2 bond with C/N–H and N–O bonds in CH3NO2 or NH2NO2 were compared using the DFT-B3LYP and MP2 methods with the 6-311++G(2d,p) and aug-cc-pVTZ basis sets. The results show that such fields have a minor effect on the C–N or C–H bond but a major effect on the N–O bond in CH3NO2, while in NH2NO2 electric fields affect the N–N bond greatly but the N–O or N–H bond only slightly. Thus, in CH3NO2, oxygen transfer or unimolecular isomerization to methyl nitrite might precede breaking of the C–N bond in the initial stages of decomposition, and the N–O bond could be the trigger bond in electric fields. In NH2NO2, however, N–N bond rupture may be preferential in an electric field and, consequently, the N–N bond might always be the real trigger bond. Atoms in molecules and natural bond orbital delocalization analyses, together with examination of shifts in electron density and frequencies support the above viewpoints. Forty-eight good linear correlations were found along the different field orientations at different levels of theory, including those between field strength (E) and changes in N−O/N−N bond length (ΔR N−O/N−N), ρ (N−O/N−N) values [Δρ (N−O/N−N), or stretching frequencies of the N−O/N−N bond (ΔυN−O/N−N).

External electric fields have a major effect on the N–O or N–N bond inCH3NO2 or NH2NO2 , leading to a possible N–O trigger bond inCH3NO2 or a real N–N trigger bond in NH2NO2 in an electric field




Similar content being viewed by others
References
Tasker D (1985) The properties of condensed explosives for electromagnetic energy coupling. NSWC TR 85–360
Demske D (1982) The experimental aspects of coupling electrical energy into a dense detonation wave: Part 1. NSWC TR 79–143
Piehler T, Hummer C, Benjamin R, Summers E, McNesby K, Boyle V (2013) Preliminary study of coupling electrical energy to detonation reaction zone of primasheet-1000 explosive. 27th International Symposium on Ballistics. Freiburg, Germany, pp 22–26
Li JS (2010) A quantitative relationship for the shock sensitivities of energetic compounds based on X–NO2 (X = C, N, O) bond dissociation energy. J Hazard Mater 180:768–772
Cao CZ, Gao S (2007) Two dominant factors influencing the impact sensitivities of nitrobenzenes and saturated nitro compounds. J Phys Chem B 111:12399–12402
Zhao J, Cheng XL, He B et al (2006) Neural networks study on the correlation between impact sensitivity and molecular structures for nitramine explosives. Struct Chem 17:501–507
Song XS, Cheng XL, Yang XD et al (2006) Relationship between the bond dissociation energies and impact sensitivities of some nitro-explosives. Propell Explos Pyrot 31:306–310
Zhang CY, Shu YJ, Huang YG et al (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109:8978–8982
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106:1770–1783
Tan BS, Long XP, Peng RF et al (2010) Two important factors influencing shock sensitivity of nitro compounds: bond dissociation energy of X–NO2 (X = C, N, O) and Mulliken charges of nitro group. J Hazard Mater 183:908–912
Politzer P, Murray JS, Concha MC et al (2007) Effects of electric fields upon energetic molecules: nitromethane and dimethylnitramine. Cent Eur J Energ Mat 4:3–21
Politzer P, Murray JS, Lane P (2009) Computational determination of effects of electric fields upon “trigger linkages” of prototypical energetic molecules. Int J Quant Chem 109:534–539
Politzer P, Murray JS (2009) Computed effects of electric fields upon the C–NO2 and N–NO2 bonds of nitromethane and dimethylnitramine. Int J Quant Chem 109:3–7
Li Z, Huang H, Zhang T et al (2014) First-principles study of electric field effects on the structure, decomposition mechanism, and stability of crystalline lead styphnate. J Mol Model 20:2072–2079
Ren WZ, Wang ZS (2004) Explosive Theory and Practice. China North Chemical Industries Corp Press, Nanjing, China
Pedley JB, Naylor RD, Kirby SP (1986) Thermochemical data of organic compounds, 2nd edn. Chapman, New York
Margetis D, Kaxiras E, Elstner M et al (2002) Electronic structure of solid nitromethane: effects of high pressure and molecular vacancies. J Chem Phys 117:788–799
Citroni M, Bini R, Pagliai M et al (2010) Nitromethane decomposition under high static pressure. J Phys Chem B 114:9420–9428
Han SP, van Duin ACT, Goddard WA et al (2011) Thermal decomposition of condensed-phase nitromethane from molecular dynamics from ReaxFF reactive dynamics. J Phys Chem B 115:6534–6540
Guo F, Cheng X, Zhang H (2012) Reactive molecular dynamics simulation of solid nitromethane impact on (010) surfaces induced and nonimpact thermal decomposition. J Phys Chem A 116:3514–3520
Taylor HA, Vesselovsky VV (1934) The thermal decomposition of nitromethane. J Phys Chem 39:1095–1102
Cottrell TL, Graham TE, Reid TJ (1951) The thermal decomposition of nitroethane and 1-nitropropane. Trans Faraday Soc 47:1089–1092
Chang J, Lian P, Wei DQ et al (2010) Thermal decomposition of the solid phase of nitromethane: ab initio molecular dynamics simulations. Phys Rev Lett 105:188302–188305
Hu WF, He TJ, Chen DM et al (2002) Theoretical study of the CH3NO2 unimolecular decomposition potential energy surface. J Phys Chem A 106:7294–7303
Bardo RD (1989) Ninth symposium (international) on detonation. Office of Naval Research, Arlington, p 235
Bardo RD (1985) Eighth symposium (international) on detonation. Naval Surface Weapons Center, White Oak, p 855
Zhou SQ, Zhao FQ, Ju XH et al (2010) A density functional theory study of adsorption and decomposition of nitroamine molecules on the Al(111) surface. J Phys Chem C 114:9390–9397
Ju GZ, Ju Q (1991) The theoretically thermodynamic and kinetic studies on the scission and rearrangement of NH2NO2. Chem J Chin Univ 12:1669–1671
Yim WL, Liu ZF (2001) Application of ab initio molecular dynamics for a priori elucidation of the mechanism in unimolecular decomposition: the case of 5-Nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO). J Am Chem Soc 123:2243–2250
Brill TB, James KJ (1993) Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem Rev 93:2667–2692
Murray JS, Concha MC, Politzer P (2009) Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C–NO2/N–NO2 bond dissociation energies. Mol Phys 107:89–97
Politzer P, Lane P, Murray JS (2011) Computational characterization of a potential energetic compound: 1, 3, 5, 7-tetranitro-2, 4, 6, 8-tetraazacubane. Cent Eur J Energ Mater 8(1):39–52
Frisch MJ, Trucks GW, Schlegel HB et al (2003) Gaussian 03, Revision B.03. Gaussian, Inc, Pittsburgh
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
Biegler-König FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comput Chem 3:317–328
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Scheiner S, Kar T (2002) Red- versus blue-shifting hydrogen bonds: are there fundamental distinctions? J Phys Chem A 106:1784–1789
Grimme S (1996) Theoretical bond and strain energies of molecules derived from properties of the charge density at bond critical points. J Am Chem Soc 118:1529–1534
Politzer P, Lane P (1996) Comparison of density functional calculations of C − NO2, N − NO2 and C − NF2 dissociation energies. J Mol Struct (Theochem) 388:51–55
Wiener JJM, Politzer P (1998) Comparison of various density functional methods for computing bond dissociation energies. J Mol Struct (Theochem)427:171–174
Ethical Statement
We have full control of all primary data and we agree to allow the journal to review all the data if requested. We confirm the validity of the results from this manuscript. We have no financial relationship with the organization that sponsored the research, or authorships. The manuscript has not been submitted to more than one journal for simultaneous consideration, and it has not been published previously (partly or in full). This study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time. No data have been fabricated or manipulated (including images).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
All the molecular structures, geometrical parameters, AIM results, and frequencies in the different field orientations at the different levels of theory are in Appendix A. (DOC 3121 kb)
About this article
Cite this article
Ren, Fd., Cao, Dl., Shi, Wj. et al. A theoretical prediction of the possible trigger linkage of CH3NO2 and NH2NO2 in an external electric field. J Mol Model 21, 145 (2015). https://doi.org/10.1007/s00894-015-2699-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2699-9