Abstract
4-[N-(2-cyanoethyl)-N-ethylamino]-4′-nitroazo-benzene (disperse orange 25, DO25) is one of the main components in dyeing wastewater. In this work, supercritical water oxidation (SCWO) process of DO25 has been investigated using the molecular dynamic simulations based on the reactive force field (ReaxFF). For the SCWO system, the effects of temperature, the molecular ratio of DO25, O2 and H2O as well as the reaction time have been analyzed. The simulated results showed that the aromatic rings in DO25 could be attacked by hydroxyl radical, oxygen molecule, and hydroxyl radical together with oxygen molecule, respectively, which caused the aromatic ring-opening reaction to happen mainly through three different pathways. The hydroxyl radicals were mainly from water clusters and H2O2 (which was produced from oxygen molecules reacting with water clusters). However, for the SCW system as comparison, the aromatic rings in DO25 could be attacked by hydroxyl radical only, and the OH radicals just come from water clusters. During the DO25 SCWO degradation process, we also found that N elements in one DO25 molecule were difficult to be converted into environmentally friendly N2 molecules because of steric hindrance, but increasing the number of DO25 molecules could improve the possibility for the connection of N elements, thus promoting N element converting into N2. Extending reaction time could also improve N elements in DO25 to transform into N2 rather than carbonitride.

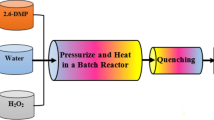
The processes of making DO25 wastewater by SCWO into clean water











Similar content being viewed by others
References
Kummerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere 45:957–969
Modell M (1982) Processing methods for the oxidation of organics in supercritical water. Google patents
Gong YM, Wang SZ, Tang XY, Xu DH, Ma HH (2014) Supercritical water oxidation of acrylic acid production wastewater. Environ Technol 35:907–916
Marrone PA (2013) Supercritical water oxidation-current status of full-scale commercial activity for waste destruction. J Supercrit Fluids 79:283–288
Shin YH, Lee HS, Veriansyah B, Kim J, Kim DS, Lee HW, Youn YS, Lee YW (2012) Simultaneous carbon capture and nitrogen removal during supercritical water oxidation. J Supercrit Fluids 72:120–124
Wang Q, Lv YK, Zhang R, Jicheng BI (2013) Treatment of cotton printing and dyeing wastewater by supercritical water oxidation. Desalin Water Treat 51:7025–7035
Han LN, Zhang R, Bi JC, Cheng LM (2011) Pyrolysis of coal-tar asphaltene in supercritical water. J Anal Appl Pyrolysis 91:281–287
Gong WJ, Li F, Xi DL (2008) Oxidation of industrial dyeing wastewater by supercritical water oxidation in transpiring-wall reactor. Water Environ Res 80:186–192
Perez IV, Rogak S, Branion R (2004) Supercritical water oxidation of phenol and 2,4-dinitrophenol. J Supercrit Fluids 30:71–87
Sogut OO, Akgun M (2007) Treatment of textile wastewater by SCWO in a tube reactor. J Supercrit Fluids 43:106–111
Dong X, Wang Y, Li X, Yu Y, Zhang M (2014) Process simulation of laboratory wastewater treatment via supercritical water oxidation. Ind Eng Chem Res 53:7723–7729
Li H, Oshima Y (2005) Elementary reaction mechanism of methylamine oxidation in supercritical water. Ind Eng Chem Res 44:8756–8764
Fujii T, Hayashi R, Kawasaki S, Suzuki A, Oshima Y (2011) Water density effects on methanol oxidation in supercritical water at high pressure up to 100 MPa. J Supercrit Fluids 58:142–149
Chang SJ, Liu YC (2007) Degradation mechanism of 2,4,6-trinitrotoluene in supercritical water oxidation. J Environ Sci 19:1430–1435
Wang SZ, Guo Y, Wang LA, Wang YZ, Xu DH, Ma HH (2011) Supercritical water oxidation of coal: investigation of operating parameters’ effects, reaction kinetics and mechanism. Fuel Process Technol 92:291–297
Savage PE, Yu JL, Stylski N, Brock EE (1998) Kinetics and mechanism of methane oxidation in supercritical water. J Supercrit Fluids 12:141–153
Garcia-Jarana MB, Kings I, Sanchez-Oneto J, Portela JR, Al-Duri B (2013) Supercritical water oxidation of nitrogen compounds with multi-injection of oxygen. J Supercrit Fluids 80:23–29
Benjamin KM, Savage PE (2005) Supercritical water oxidation of methylamine. Ind Eng Chem Res 44:5318–5324
Kim K, Son SH, Kim K, Kim K, Kim YC (2010) Environmental effects of supercritical water oxidation (SCWO) process for treating transformer oil contaminated with polychlorinated biphenyls (PCBs). Chem Eng J 165:170–174
Ngo TT, Liotta CL, Eckert CA, Kazarian SG (2003) Supercritical fluid impregnation of different Azo-dyes into polymer: in situ Uv/Vis spectroscopic study. J Supercrit Fluids 27:215–221
van Duin ACT, Dasgupta S, Lorant F, Goddard WA (2001) ReaxFF: a reactive force field for hydrocarbons. J Phys Chem A 105:9396–9409
Chenoweth K, van Duin ACT, Dasgupta S, Goddard WA (2009) Initiation mechanisms and kinetics of pyrolysis and combustion of JP-10 hydrocarbon jet fuel. J Phys Chem A 113:1740–1746
Chenoweth K, van Duin ACT, Goddard WA (2008) ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J Phys Chem A 112:1040–1053
Salmon E, Behar F, Lorant F, Hatcher PG, Marquaire PM (2009) Early maturation processes in coal. Part 1: pyrolysis mass balance and structural evolution of coalified wood from the morwell brown coal seam. Org Geochem 40:500–509
Nielson KD, van Duin ACT, Oxgaard J, Deng WQ, Goddard WA (2005) Development of the ReaxFF reactive force field for describing transition metal catalyzed reactions, with application to the initial stages of the catalytic formation of carbon nanotubes. J Phys Chem A 109:493–499
Chen B, Wei XY, Yang ZS, Liu C, Fan X, Qing Y, Zong ZM (2012) ReaxFF reactive force field for molecular dynamics simulations of lignite depolymerization in supercritical methanol with lignite-related model compounds. Energy Fuel 26:984–989
Zhang JL, Weng XX, Han Y, Li W, Cheng JY, Gan ZX, Gu JJ (2012) The effect of supercritical water on coal pyrolysis and hydrogen production: a combined ReaxFF and DFT study. Fuel 108:682–690
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Salmon E, van Duin ACT, Lorant F, Marquaire PM, Goddard WA III (2009) Early maturation processes in coal. Part 2: reactive dynamics simulations using the ReaxFF reactive force field on morwell brown coal structures. Org Geochem 40:1195–1209
Jin FM, Kishita A, Moriya T, Enomoto H (2001) Kinetics of oxidation of food wastes with H2O2 in supercritical water. J Supercrit Fluids 19:251–262
Bermejo MD, Cocero MJ (2006) Supercritical water oxidation: a technical review. AIChE J 52:3933–3951
Koda S, Maeda K, Sugimoto K, Sugiyama M (2006) Oxidation reactions of solid carbon in supercritical water. Combust Sci Technol 178:487–507
Koda S, Kanno N, Fujiwara H (2001) Kinetics of supercritical water oxidation of methanol studied in a CSTR by means of Raman spectroscopy. Ind Eng Chem Res 40:3861–3868
Phenix BD, Dinaro JL, Tatang MA, Tester JW, Howard JB, McRae GJ (1998) Incorporation of parametric uncertainty into complex kinetic mechanisms: application to hydrogen oxidation in supercritical water. Combust Flame 112:132–146
Acknowledgments
The project was supported by the National High-Tech Research and Development Program of China (2011AA05A201), National Natural Science Foundation of China (21106094, 21206021), International Science & Technology Cooperation Program of China (2013DFG42680), the Program for Changjiang Scholars, Innovative Research Team in University (IRT1161), and Tianjin Science Foundation for Youths, China (12JCQNJC03100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Gu, J., Han, Y. et al. Analysis of degradation mechanism of disperse orange 25 in supercritical water oxidation using molecular dynamic simulations based on the reactive force field. J Mol Model 21, 54 (2015). https://doi.org/10.1007/s00894-015-2603-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2603-7