Abstract
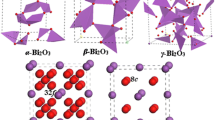
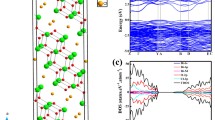
The effect of N doping on the crystal structure, electronic, and optical properties of α-Bi2O3 and β-Bi2O3 has been studied in detail based with first principle calculations. The crystallographic features of Bi2O3 polymorphs are not substantially changed through N doping, whereas charge transfer from Bi to N results in large variations of charge density distribution. N-doped β-Bi2O3 exhibits improved thermal stability due to stronger Bi-N covalent bonds and lower defect formation energy, and the convenient preparative access agrees well with experimental observations. Calculated band structures and optical properties indicate that N doping does not induce major band gap narrowing, but leads to the presence of isolated bands above the VBM induced by N 2p for both α-Bi2O3 and β-Bi2O3 which induce large red-shifts of their visible light absoprtion properties. These isolated bands act as acceptor levels and facilitate electron transition under visible light illumination through introduction of steps between VB and CB, thereby rendering the materials quite promising for photocatalytic applications.





Similar content being viewed by others
References
Chen XB, Mao SS (2007) Chem Rev 107:2891–2959
Linsebigler AL, Lu G, Yates JT Jr (1995) Chem Rev 95:735–758
Wang F, Cao K, Zhang Q, Gong XD, Zhou Y (2014) J Mol Model 20:2506
Leontie L, Caraman M, Delibas M, Rusu GI (2001) Mater Res Bull 36:1629–1637
Muruganandham M, Amutha R, Lee GJ, Hsieh SH, Wu JJ, Sillanpää MJ (2012) Phys Chem C 116:12906–12915
Huang Q, Zhang S, Cai C, Zhou B (2011) Mater Lett 65988–65990
Cheng H, Huang B, Lu J, Wang Z, Xu B, Qin X, Zhang X, Dai Y (2010) Phys Chem Chem Phys 12:15468–15475
Romanov AN, Fattakhova ZT, Rufov YN, Shashkin DP (2001) Kinet Catal 42:275–280
Xia N, Yuan D, Zhou T, Chen J, Mo S, Liu Y (2011) Mater Res Bull 46:687–691
Dai G, Liu S, Liang Y (2014) J Alloys Compd 608:44–48
Lu YG, Yang YC, Xiang YZ, Liu SY (2012) J Inorg Mater 27:643–648
Jun S, Yuan G, Hao WC, Jing X, Ju HX, Wang L, Feng HF, Wang TM (2014) Chin Phys B 23:038103
Li M, Li F, Yin PG (2014) Chem Phys Lett 601:92–97
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Batzill M, Morales EH, Diebold U (2006) Phys Rev Lett 96:026103
Valentin CD, Pacchioni G, Selloni A (2004) Phys Rev B 70085116
Serpone N (2006) J Phys Chem B 110:24287–24293
Perdew JP, Wang Y (1992) Phys Rev B 45:13244
Vanderbilt D (1990) Phys Rev B 41:7892
Segall MD, Lindan PJD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) J Phys: Condensed Mat 14:2717
Fischer TH, Almlof J (1992) J Phys Chem 96:9768–9774
Dong H, Chen G, Sun JX, Li CM, Yu YG, Chen DH (2013) Appl Catal B: Environmental 134:46–54
Lai KR, Zhu YT, Lu JB, Dai Y, Huang BB (2013) Comp Mater Sci 67:88–92
Wang J, Tafen DN, Lewis JP, Hong ZL, Manivannan A, Zhi MJ, Li M, Wu NQ (2009) J Am Chem Soc 131:12290–12297
Han XP, Shao GS (2011) J Phys Chem C 115:8274–8282
Yu JG, Zhou P, Li Q (2013) Phys Chem Chem Phys 151:2040–12047
Harwig HA (1978) Allg Chem 444:167–177
Wu GH, Zheng SK, Wu PF, Su J, Liu L (2013) Solid State Commun 163:7–10
Yang KS, Dai Y, Huang BB (2007) J Phys Chem C 111:12086–12090
Dashora A, Patel N, Kothari DC, Ahuja BL, Miotello A (2014) Sol Energ Mat Sol C 125:120–126
Acknowledgments
We thank the financial support by the National Natural Science Foundation of China (51102245), Sichuan Youth Science and Technology Foundation (2013JQ0034), the Innovative Research Team of Sichuan Provincial Education Department (2012XJZT002), Scientific Research Staring Project of SWPU (2014QHZ020, 2014PYZ012), and Sichuan Provincial Colleges’ Sate Key Laboratory of Oil and Gas Reservoir Project (x151514kcl17)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Cao, K., Wu, Y. et al. Electronic and optical properties of N-doped Bi2O3 polymorphs for visible light-induced photocatalysis. J Mol Model 21, 48 (2015). https://doi.org/10.1007/s00894-015-2596-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2596-2