Abstract
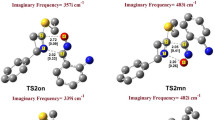
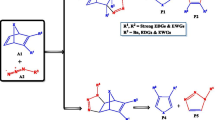
In this work we used density functional theory (DFT) B3LYP/6-31G*(d) to study the stoichiometric reaction between the product (1S,3R,8S)-2,2-dichloro-3,7,7,10-tetramethyltricyclo[6,4,0,01.3]dodec-9-ene (referred to here as P1) and dibromocarbene. We have shown that P1 behaves as a nucleophile, while dibromocarbene behaves as an electrophile; that the chemical potential of dibromocarbene is superior to that of P1 in absolute terms; and that P1 reacts with an equivalent quantity of dibromocarbene to produce two products: (1S,3R,8R,9S,11R)-10,10-dibromo-2,2-dichloro-3,7,7,11-tetramethyltetracyclo[6,5,0,01.3,09.11] tridecane (referred to here as P2) and (1S,3R,8R,9R,11S)-10,10-dibromo-2,2-dichloro-3,7,7,11-tetramethyltetracyclo[6,5,0,01.3,09.11] tridecane (referred to here as P3). P2 and P3 are formed at the α and β sides, respectively, of the C2 = C3 double bond of P1. This reaction is exothermic, stereoselective and chemospecific, and is controlled by charge transfer. Regioselectivity of the reaction was interpreted using the Lee-Yang-Parr functional.




Similar content being viewed by others
References
Dakir M, Auhmani A, Ait Itto MY, Mazoir N, Akssira M, Pierrot M, Benharref A (2004) Optimisation of allylic oxidation of (1S,3R,8R)-2,2-dichloro-3,7,7,10-tetramethyltricyclo-[6.4.0.01,3] dodec-9-ene. Synth Commun 34(11):2001–2008
Auhmani A, Kossareva E, Eljamili H, Reglier M, Pierrot M, Benharref A (2002) Regiospecific synthesis of a new chiral N-substituted pyrazole using a sesquiterpene hydrocarbon. Synth Commun 32(5):707–715
Eljamili H, Auhmani A, Dakir M, Lassaba E, Benharref A, Pierrot M, Chiaroni A, Riche C (2002) Oxydation et addition des dihalocarbènes sur le β-himachalène. Tetrahedron Lett 43:6645–6648
Auhmani A, Kossareva E, Lassaba E, Réglier M, Pierrot M and Benharref A (1999) Cyclopropanation reactions on α-cis-himachalene and a β-himachalene. Acta Cryst C 55: IUC9900055
Oukhrib A, Benharref A, Saadi M, Berraho M and El Ammari L (2013) (1S,3R,8R,9S,11R)-10,10-dibromo-2,2-dichloro-3,7,7,11-tetramethyltetracyclo-[6.5.0.01,3.09,11] tridecane. Acta Cryst E 69:o739
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the local electrophilicity of organic molecules. Understanding the regioselectivity on Diels-Alder reactions. J Phys Chem A 106:6871–6875
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423
Pérez P, Domingo LR, Duque-Noreña M, Chamorro E (2009) A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J Mol Struct Theochem 895:86–91
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793–1874
Chandra AK, Nguyen MT (2002) Use of local softness for the interpretation of reaction mechanisms. Int J Mol Sci 3:310–323
Pearson RG, Songstad J (1967) Application of the principle of hard and soft acids and bases to organic chemistry. J Am Chem Soc 89:1827–1836
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Gaussian 09 (2009) Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman,JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian, Inc., Wallingford CT
Lee CT, Yang WT, Parr RG (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev B 37:785–789
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA (2001) 6-31G* Basis set for third-row atoms. J Comp Chem 22:976–984
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494
Parr RG, Szentpály VL, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. Theor Study J Org Chem 73:4615–4624
Domingo LR, Sáez JA, Arnó M (2014) A DFT study on the NHC catalysed Michael addition of enols to α, β-unsaturated acyl-azoliums. A base catalysed C–C bond-formation step. Org Biomol Chem 12(6):895–890. doi:10.1039/c3ob41924j
Domingo LR, Aurell MJ, Pérez P (2014) The mechanism of ionic Diels-Alder reactions. A DFT study of the oxa-Povarov reaction. RSC Adv 4:16567–16577. doi:10.1039/c3ra47805j
Domingo LR, Aurell MJ, Sáez JA, Pérez P (2014) Understanding the mechanism of the Povarov reaction. A DFT study. RSC Adv 4:25268–25278. doi:10.1039/c4ra02916j
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428. doi:10.1039/c4ra04280h
Zeroual A, El Idrissi M, Benharref A, El Hajbi A (2014) Theoretical study of regioselectivity and stereoselectivity of condensation of β-himachalene with dichlorocarbene using density functional theory (DFT). Int J Innovation Appl Study 5:120–130
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013
Rights and permissions
About this article
Cite this article
Zeroual, A., Benharref, A. & El Hajbi, A. Theoretical study of stereoselectivity of the [1 + 2] cycloaddition reaction between (1S,3R,8S)-2,2-dichloro-3,7,7,10-tetramethyltricyclo[6,4,0,01.3]dodec-9-ene and dibromocarbene using density functional theory (DFT) B3LYP/6-31G*(d). J Mol Model 21, 44 (2015). https://doi.org/10.1007/s00894-015-2594-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2594-4