Abstract
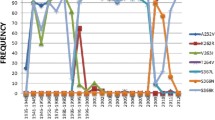
Following the influenza A (H1N1) pandemic in Mexico and around the world in 2009, the numbers of oseltamivir-resistant clinical cases have increased through a mechanism that remains unclear. In this work, we focus on studying the mutated NA structures ADA71175 (GenBank) and 3CKZ (PDB ID). Recently crystallized NA (PDB ID: 3NSS) was used as a wild-type structure and template to construct the three-dimensional (3D) structure of ADA71175. Then, the NA mutants and 3NSS natives as well as their refined monomer structures as determined through MD simulations (snapshots at 50 ns) were used as models to perform a docking study using a set of aryl-oseltamivir derivatives. These aryl-oseltamivir derivatives have better recognition properties than oseltamivir because of cation–π interactions with a cluster of Arg residues (118, 292, and 371) at the binding site. This cluster of Arg residues represents a potential binding site for aryl-oseltamivir derivatives that are potentially new NA inhibitors.






Similar content being viewed by others
References
Zhong S, MacKerell AD Jr (2007) J Chem Inf Model 47:2303–2315
Navarro-Polanco RA, Moreno Galindo EG, Ferrer-Villada T, Arias M, Rugby JR, Sanchez-Chapula JA, Tristani-Firouzi M (2011) J Physiol 589(Pt 7):1741–1753. doi:10.1113/jphysiol.2010.204107
Vijayan R, Sahai MA, Czajkowski T, Biggin PC (2010) Phys Chem Chem Phys 12:14057–14066
Demina A, Varughese KI, Barbot J, Forman L, Beutler E (1998) Blood 92:647–652
Nguyen AP, Downard KM (2013) Analyst 138:1787–1793
Chen LF, Dailey NJ, Rao AK, Fleischauer AT, Greenwald I, Deyde VM, Moore ZS, Anderson DJ, Duffy J, Gubareva LV, Sexton DJ, Fry AM, Srinivasan A, Wolfe CR (2011) J Infect Dis 203:838–846
Longtin J, Patel S, Eshaghi A, Lombos E, Higgins R, Alexander D, Olsha R, Doyle J, Tran D, Sarabia A, Lee C, Bastien N, Li Y, Low D, Boivin G, Gubbay J (2011) J Clin Virol 50:257–261
Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gamblin SJ (2008) Nature 453:128–1261
Woods CJ, Malaisree M, Pattarapongdilok N, Sompornpisut P, Hannongbua S, Mulholland A (2012) J Biochem 51:4364–4375
Basha SH, Prasad RN (2012) BMC Res Notes 5:105
Sheu TG, Fry AM, Garten RJ, Deyde VM, Shwe T, Bullion L, Peebles PJ, Li Y, Klimov AI, Gubareva LV (2011) J Infect Dis 203:13–71
Esposito S, Molteni CG, Daleno C, Valzano A, Fossali E, Da Dalt L, Cecinati V, Bruzzese E, Giacchino R, Giaquinto C, Galeone C, Lackenby A, Principi N (2010) Virol J 7:202
Govorkova EA, Ilyushina NA, Marathe BM, McClaren JL, Webster RG (2010) J Virol 84:8042–8050
Zepeda HM, Perea-Araujo L, Zarate-Segura PB, Vázquez-Pérez JA, Miliar-García A, Garibay-Orijel C, Domínguez-López A, Badillo-Corona JA, López-Orduña E, García-González OP, Villaseñor-Ruíz I, Ahued-Ortega A, Aguilar-Faisal L, Bravo J, Lara-Padilla E, García-Cavazos RJ (2010) J Clin Virol 48:36–39
Tolentino-Lopez L, Segura-Cabrera A, Reyes-Loyola P, Zimic M, Quiliano M, Briz V, Muñoz-Fernández A, Rodríguez-Pérez M, Ilizaliturri-Flores I, Correa-Basurto J (2013) Biopolymers 99(1):10–21. doi:10.1002/bip.22130
Loyola PKR, Campos-Rodríguez R, Bello M, Rojas-Hernández S, Zimic M, Quiliano M, Briz V, Ángeles Muñoz-Fernández M, Tolentino-López L, Correa-Basurto J (2013) Immunol Res 56:44–60. doi:10.1007/s12026-013-8385-z
Bearman GM, Shankaran S, Elam K (2010) Drug Discov 5:152–156
Memoli MJ, Hrabal RJ, Hassantoufighi A, Eichelberger MC, Taubenberger JK (2010) Clin Infect Dis 50:1252–1255
Pizzorno A, Bouhy X, Abed Y, Boivin G (2012) Anal Chem 84(8):3725–3730. doi:10.1021/ac300291c
Shie JJ, Fang JM, Lai PT, Wen WH, Wang SY, Cheng YS, Tsai KC, Yang AS, Wong CH (2011) J Am Chem Soc 133(44):17959–17965
Gasymov OK, Abduragimov AR, Glasgow BJ (2012) Biochemistry 51(14):2991–3002. doi:10.1021/bi3002902
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Peterson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.9. Gaussian, Inc., Pittsburgh
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart E, Belew RK, Olson AJ (1998) J Comput Chem 19:1639–1662
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33–38
Rayamajhi N, Joo JC, Cha SB, Pokherl S, Shin MK, Yoo YJ, Yoo HS (2011) Microbiology 11:29
Kanibolotsky DS, Novosyl’na OV, Abbott CM, Negrutskii BS, El'skaya AV (2008) BMC Struct Biol 8:4
Meijer A, Jonges M, Abbink F, Ang W, van Beek J, Beersma M, Bloembergen P, Boucher C, Claas E, Donker G, van Gageldonk-Lafeber R, Isken L, de Jong A, Kroes A, Leenders S, van der Lubben M, Mascini E, Niesters B, Oosterheert JJ, Osterhaus A, Riesmeijer R, Riezebos-Brilman A, Schutten M, Sebens F, Stelma F, Swaan C, Timen A, Van’t Veen A, van der Vries E, Te Wierik M, Koopmans M (2011) Antivir Res 92(1):81–89
]Aoki FY, Boivin G, Roberts N (2007) Antivir Ther 12(4 Pt B):603–616
Storms AD, Gubareva LV, Su S, Wheeling JT, Okomo-Adhiambo M, Pan CY, Reisdorf E, St George K, Myers R, Wotton JT, Robinson S, Leader B, Thompson M, Shannon M, Klimov A, Fry AM (2012) Emerg Infect Dis 18(2):308–311. doi:10.3201/eid1802.111466
Kramarz P, Monnet D, Nicoll A, Yilmaz C, Ciancio B (2009) Euro Surveill 14(5):19112
Jardón-Valadez E, Ulloa-Aguirre A, Piñeiro A (2008) J Phys Chem B 112:10704–10713
Pan P, Li L, Li Y, Li D, Hou T (2013) Antivir Res 100:356–364
Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ (2006) Nature 443:45–49
Sine SM, Wang HL, Bren N (2002) J Biol Chem 277:29210–29223
Karthick V, Shanthi V, Rajasekaran R, Ramanathan K (2013) Protoplasma 250(1):197–207. doi:10.1007/s00709-012-0394-6
Peng DY, Sun Q, Zhu XL, Lin HY, Chen Q, Yu NX, Yang WC, Yang GF (2012) Bioorg Med Chem 20(22):6739–6750
Unsal-Tan O, Ozadali K, Piskin K, Balkan A (2012) Eur J Med Chem 57C:59–64
Yewale SB, Ganorkar SB, Baheti KG, Shelke RU (2012) Bioorg Med Chem Lett 22(21):6616–6620
Wang M, Qi J, Liu Y, Vavricka CJ, Wu Y, Li Q, Gao GF (2011) J Virol 85(16):8431–8435. doi:10.1128/JVI. 00638-11
Rudrawar S, Kerry PS, Rameix-Welti MA, Maggioni A, Dyason JC, Rose FJ, van der Werf S, Thomson RJ, Naffakh N, Russell RJ, von Itzstein M (2012) Org Biomol Chem 10(43):8628–8639
Acknowledgments
The work was supported by grants from ICyTDF (Instituto de Ciencia y Tecnología del Distrito Federal), CONACYT (Consejo Nacional de Ciencia y Tecnología), CYTED (Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo) and BEIFI (BECA DE ESTÍMULO INSTITUCIONAL DE FORMACIÓN DE INVESTIGADORES), SIP (secretaria de investigación y posgrado), COFAA (COMISIÓN DE OPERACIÓN Y FOMENTO DE ACTIVIDADES ACADEMICAS) from IPN (Instituto Politécnico Nacional, México).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gema, L.RS., Tolentino-Lopez, L.E., Martínez-Ramos, F. et al. Targeting a cluster of arginine residues of neuraminidase to avoid oseltamivir resistance in influenza A (H1N1): a theoretical study. J Mol Model 21, 8 (2015). https://doi.org/10.1007/s00894-014-2525-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2525-9