Abstract
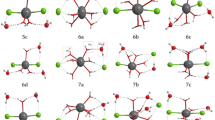
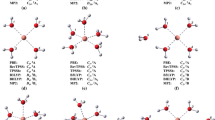
In this work we have developed a polarizable potential to study Cm(III) forming complexes with carbonate anions in liquid water. The potential was developed by employing an extension of the procedure that we used to study the hydration of lanthanoids(III) and actinoids(III). Force field performances were benchmarked against DFT results obtained by both geometry optimization and Car-Parrinello molecular dynamics. With this polarizable potential, we run extended molecular dynamics simulations in liquid water from which we were able to identify structural and dynamical properties of such systems. In particular, water exchange dynamics were analyzed in detail. We obtained an average of three water molecules in the first shell of Cm(III) that show a relatively fast exchange dynamic (faster than for bare ions). Summarizing these results, we were able to draw an analogy to the results from the lanthanoid(III) series. In particular, it seems that Cm(III) behaves more like Nd(III) than Gd(III), as one would expect based on the recent hydration results and on f orbital occupancy.







Similar content being viewed by others
References
Kemsley J (2010) With new fuel formulations reactors could extract more energy, reduce hazardous waste. Chem Eng News 88:29–31
Kaltsoyannis N, Scott P (2007) The f elements, Oxford chemistry primers. Oxford University Press, Oxford
Peppard DF, Gray PR, Markus MM (1953) The actinide—lanthanide analogy as exemplified by solvent extraction behavior. J Am Chem Soc 75:6063–6064
Wietzke R, Mazzanti M, Latour J-M, Pecaut J, Cordier P-Y, Madic C (1998) Lanthanide(III) Complexes of tripodal N-donor ligands: structural models for the species involved in solvent extraction of actinides(III). Inorg Chem 37:6690–6697
Philippini V, Vercouter T, Vitorge P (2010) Evidence of different stoichiometries for the limiting carbonate complexes across the lanthanide(III) series. J Solution Chem 39:747–769
Allard B, Kipatsi H, Liljenzin JO (1980) Expected species of uranium, neptunium and plutonium in neutral aqueous solutions. J Inorg Nucl Chem 42:1015–1027
Helm L, Merbach AE (2005) Inorganic and bioinorganic solvent exchange mechanisms. Chem Rev 105:1923–1960
D’Angelo P, Spezia R (2012) Hydration of lanthanoids(III) and actinoids(III): a theoretical/experimental saga. Chem Eur J 18:11162–11178
Persson I, D’Angelo P, De Panfilis S, Sandstrom M, Eriksson L (2008) Hydration of lanthanoid(III) ions in aqueous solution and crystalline hydrates studied by EXAFS spectroscopy and crystallography: the myth of the “gadolinium break”. Chem Eur J 14:3056–3066
Marjolin A, Gourlaouen C, Clavaguera C, Ren PY, Wu JC, Gresh N, Dognon J-P, Piquemal J-P (2012) Toward accurate solvation dynamics of lanthanides and actinides in water using polarizable force fields: from gas-phase energetics to hydration free energies. Theo Chem Acc 131:1198
Cossy C, Helm L, Powell DH, Merbach AE (1995) A change in coordination-number from 9 to 8 along the lanthanide(III) aqua ion series in solution — a neutron-diffraction study. New J Chem 19:27–35
Habenschuss A, Spedding FH (1979) The coordination (hydration) of rare earth ions in aqueous chloride solutions from x ray diffraction. I. TbCl3, DyCl3, ErCl3, TmCl3, and LuCl3. J Chem Phys 70:2797
Kuta J, Clark AE (2010) Trends in aqueous hydration across the 4f period assessed by reliable computational methods. Inorg Chem 49:7808–7817
Ciupka J, Cao-Dolg X, Wiebke J, Dolg M (2010) Computational study of lanthanide(III) hydration. Phys Chem Chem Phys 12:13215–13223
Kowall T, Foglia F, Helm L, Merbach AE (1995) Molecular dynamics simulation study of lanthanide ions Ln3+ in aqueous solution including water polarization. Change in coordination number from 9 to 8 along the series. J Am Chem Soc 117:3790–3799
Floris FM, Tani A (2001) A study of aqueous solutions of lanthanide ions by molecular dynamics simulation with ab initio effective pair potentials. J Chem Phys 115:4750
Duvail M, Spezia R, Vitorge P (2008) A dynamic model to explain hydration behaviour along the Lanthanide series. ChemPhysChem 9:693–696
Clavaguéra C, Pollet R, Soudan JM, Brenner V, Dognon J-P (2005) Molecular dynamics study of the hydration of lanthanum(III) and europium(III) including many-body effects. J Phys Chem B 109:7614–7616
Beuchat C, Hagberg D, Spezia R, Gagliardi L (2010) The hydration of lanthanide-chloride salts: a quantum chemical and classical molecular dynamics simulation study. J Phys Chem B 114:15590–15597
Villa A, Hess B, Saint-Martin H (2009) Dynamics and structure of Ln(III) − aqua ions: a comparative molecular dynamics study using ab initio based flexible and polarizable model potentials. J Phys Chem B 113:7270–7281
Meier W, Bopp P, Probst MM, Spohr E, Lin JL (1990) Molecular dynamics studies of lanthanum chloride solutions. J Phys Chem 94:4672–4682
Skanthakumar S, Antonio M, Wilson R, Soderholm L (2007) The curium aqua ion. Inorg Chem 46:3485–3491
Galbis E, Hernandez-Cobos J, Den Auwer C, Naour CL, Guillaumont D, Simoni E, Pappalardo RR, Sanchez-Marcos E (2010) Solving the hydration structure of the heaviest actinide aqua ion known: the californium(III) case. Angew Chem Int Ed 49:3811–3815
Real F, Trumm M, Schimmelpfennig B, Masella M, Vallet V (2013) Further insights in the ability of classical nonadditive potentials to model actinide ionwater interactions. J Comput Chem 34:707–719
Brendebach B, Banik NL, Marquardt CM, Rothe J, Denecke M, Geckeis H (2009) X-ray absorption spectroscopic study of trivalent and tetravalent actinides in solution at varying pH values. Radiochim Acta 97:701–708
Allen P, Bucher J, Shuh DK, Edelstein NM, Craig I (2000) Coordination chemistry of trivalent lanthanide and actinide ions in dilute and concentrated chloride solutions. Inorg Chem 39:595–601
Lindqvist-Reis P, Apostolidis C, Rebizant J, Morgenstern A, Klenze R, Walter O, Fanghanel T, Haire RG (2007) The structures and optical spectra of hydrated transplutonium ions in the solid state and in solution. Angew Chem Int Ed 46:919–922
Revel R, Den Auwer C, Madic C, David F, Fourest B, Hubert S, Le Du J, Morss LR (1999) First investigation on the L edges of the 249Cf aquo ion by X-ray absorption spectroscopy. Inorg Chem 38:4139–4141
Antonio M, Soderholm L, Williams CW, Blaudeau J-P, Bursten B (2001) Neptunium redox speciation. Radiochim Acta 89:17–25
Wiebke J, Moritz A, Cao X, Dolg M (2007) Approaching actinide(+III) hydration from first principles. Phys Chem Chem Phys 9:459–465
Hagberg D, Bednarz E, Edelstein NM, Gagliardi L (2007) A quantum chemical and molecular dynamics study of the coordination of Cm(III) in water. J Am Chem Soc 129:14136–14137
Spezia R, Jeanvoine Y, Beuchat C, Gagliardi L, Vuilleumier R (2014) Hydration properties of Cm(III) and Th(IV) combining coordination free energy profiles with electronic structure analysis. Phys Chem Chem Phys 16:5824–5832
D’Angelo P, Zitolo A, Migliorati V, Chillemi G, Duvail M, Vitorge P, Abadie S, Spezia R (2011) Revised ionic radii of lanthanoid(III) ions in aqueous solution. Inorg Chem 50:4572–4579
D’Angelo P, Martelli F, Spezia R, Filipponi A, Denecke MA (2013) Hydration properties and ionic radii of actinide(III) ions in aqueous solution. Inorg Chem 52:10318–10324
Duvail M, Vitorge P, Spezia R (2009) Building a polarizable pair interaction potential for lanthanoids(III) in liquid water: a molecular dynamics study of structure and dynamics of the whole series. J Chem Phys 130:104501
Duvail M, Martelli F, Vitorge P, Spezia R (2011) Polarizable interaction potential for molecular dynamics simulations of actinoids(III) in liquid water. J Chem Phys 135:044503
Martelli F, Jeanvoine Y, Vercouter T, Beuchat C, Vuilleumier R, Spezia R (2014) Hydration properties of Lanthanoid(III) carbonates complexes in liquid water by polarizable molecular dynamics simulations. Phys Chem Chem Phys 16:3693–3705
Jeanvoine Y, Miro P, Martelli F, Cramer CJ, Spezia R (2012) Electronic structure and bonding of lanthanoid(III) carbonates. Phys Chem Chem Phys 14:14822–14831
Runde W, Neu MP, Van Pelt C, Scott BL (2000) Single crystal and solution complex structure of Nd(CO3)4 5−. The first characterization of a mononuclear lanthanide(III) carbonato complex. Inorg Chem 39:1050–1051
Janicki R, Starynowicz P, Mondry A (2011) Lanthanide carbonates. Eur J Inorg Chem 2011:3601–3616
Sherry HS, Marinsky JA (1963) Carbonate and bicarbonate complexes of neodymium and europium. Inorg Chem 3:330–335
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474
Vercouter T, Vitorge P, Trigoulet N, Giffaut E, Moulin C (2005) Eu(CO3)(3) (3-) and the limiting carbonate complexes of other M3+ f-elements in aqueous solutions: a solubility and TRLFS study. New J Chem 29:544–553
Philippini V, Vercouter T, Aupiais J, Topin S, Ambard C, Chaussé A, Vitorge P (2008) Evidence of different stoichiometries for the limiting carbonate complexes across the lanthanide(III) series: a capillary electrophoresis-mass spectrometry study. Electrophoresis 29:2041–2050
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Kuechle W, Dolg M, Stoll H, Preuss H (1994) Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J Chem Phys 100:7535
Cao X, Dolg M, Stoll H (2003) Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J Chem Phys 118:487
Cao X, Dolg M (2004) Segmented contraction scheme for small-core actinide pseudopotential basis sets. J Molec Struct (Theochem) 673:203–209
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian, Inc, Wallingford, CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43:1993–2006
Spezia R, Tournois G, Tortajada J, Cartailler T, Gaigeot M-P (2006) Toward a DFT-based molecular dynamics description of Co(II) binding in sulfur rich peptides. Phys Chem Chem Phys 8:2040–2050
Spezia R, Bresson C, Den Auwer C, Gaigeot M-P (2008) Solvation effects on structure and dynamics of Co(III)-cysteine complexes in water: a DFT-based molecular dynamics study. J Phys Chem B 112:6490–6499
Ayala R, Spezia R, Vuilleumier R, Martinez JM, Pappalardo RR, Sanchez Marcos E (2010) An ab initio molecular dynamics study on the hydrolisis of Po(IV) aquaion in water. J Phys Chem B 114:12866–12874
CPMD libraries freely available at www.cpmd.org/ for download
Kleinman L, Bylander DM (1982) Efficacious form for model pseudopotentials. Phys Rev Lett 48:1425–1428
Nose S (1984) A molecular-dynamics method of simulations in the canonical ensemble. Mol Phys 52:255–268
Martyna GJ, Klein ML, Tuckerman M (1992) Nosé-Hoover chains: the canonical ensemble via continuous dynamics. J Chem Phys 97:2635
Hutter J, Alavi A, Deutsch T, Bernasconi M, Goedecker S, Marx D, Tuckerman MA, Parrinello M (eds) (2004) CPMD, version 3.12, IBM Research Division. IBM Corp and Max Planck Institute, Stuttgart, Germany
Duvail M, Souaille M, Spezia R, Cartailler T, Vitorge P (2007) Pair interaction potentials with explicit polarisation for molecular dynamics simulations of La3+ in bulk water. J Chem Phys 127:034503
Martelli F, Vuilleumier R, Simonin J-P, Spezia R (2012) Varying the charge of small cations in liquid water: structural, transport and thermodynamical properties. J Chem Phys 137:164501
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) Numerical-integration of Cartesian equations of motion of a system with constraints — molecular-dynamics of N-alkanes. J Comput Phys 23:327–341
Thole BT (1981) Molecular polarizabilities calculated with a modified dipole interaction. Chem Phys 59:341–350
van Duijnen P, Swart M (1998) Molecular and atomic polarizabilities: Thole’s model revisited. J Phys Chem A 102:2399–2407
Sprik M (1991) Computer simulation of the dynamics of induced polarization fluctuations in water. J Phys Chem 95:2283–2291
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926
Weiner SJ, Kollman PA, Case DA, Singh C, Ghio C, Alagona G, Profeta S Jr, Weine PA (1984) The comparative roles of the proton-acceptor properties of amide and carboxyl groups in influencing crystal packing patterns: doubly vs. singly hydrogen-bonded systems in N-acylamino acids and in other amide-acid crystals. J Am Chem Soc 106:765–767
Souaille M, Loirat H, Borgis D, Gaigeot M-P (2009) MDVRY: a polarizable classical molecular dynamics package for biomolecules. Comput Phys Commun 180:276–301
Hutchinson F, Wilson M, Madden PA (2001) A unified description of MCl3 systems with a polarizable ion simulation model. Mol Phys 99:811–824
Salanne M, Simon C, Turq P, Madden PA (2008) Calculation of activities of ions in molten salts with potential application to the pyroprocessing of nuclear waste. J Phys Chem B 112:1177–1183
Okamoto Y, Suzuki S, Shiwaku H, Ikeda-Ohno A, Yaita T, Madden PA (2010) Local coordination about La3+ in molten LaCl3 and its mixtures with alkali chlorides. J Phys Chem A 114:4664–4671
Impey RW, Madden PA, McDonald IR (1983) Hydration and mobility of ions in solution. J Phys Chem 87:5071–5083
Acknowledgments
We thank ANR 2010 JCJC 080701 ACLASOLV (Actinoids and Lanthanoids Solvation) for grant support and Grand Equipement National de Calcul Intensif (GENCI) (grant x2013071870) for generous allocation of computing time. We thank Mrs. Elizabeth A. Kish for careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013
Rights and permissions
About this article
Cite this article
Spezia, R., Jeanvoine, Y. & Vuilleumier, R. Developing polarizable potential for molecular dynamics of Cm(III)-carbonate complexes in liquid water. J Mol Model 20, 2398 (2014). https://doi.org/10.1007/s00894-014-2398-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2398-y