Abstract
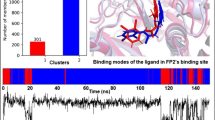
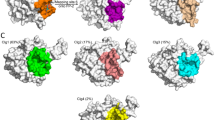
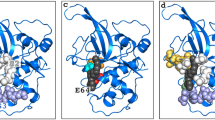
Following the increasing reports of human toxicity and plasmodium resistance to artemisinin and its derivatives, falcipain-2 (FP-2) is now emerging as the choice antimalarial drug target. Coincidentally, FP-2 is the in vivo target of naturally occurring, therapeutically safe flavonoids (stenopalustroside, myricetin, and fisetin) and symplostatin (symplostatin 4) compounds known to exhibit potent in vitro and in vivo antiplasmodial actions. Here, the structural bases for their inhibitory actions have been studied using molecular dynamics simulation. Myricetin and fisetin act as proton transfer tunnel breakers by inserting between His174 and Cys42, which are key active site residues of FP-2, stenopalustroside inhibits the polarization of His174 by Asn173; a major preparatory step for Cys42/His174 proton transfer process. The roles of flavonoids are favored by T-shaped pi–pi interactions with His174. Symplostatin 4 inserts its methyl-methoxylpyrrolinone moiety into the active site where its proton acceptor function prepares Cys42 for nucleophilic attack on the Michael α,β-unsaturated bonds on its 4(S)-amino-2(E)-pentenoate moiety. Further analyses of the structures identified a unique bridge formed on FP-2 active site groove by stenopalustroside and symplostatin 4 during interaction with the sub-site I of FP-2, whereas fisetin preferentially interacts with sub-site II and myricetin interacts with sub–site III residues. Ultimately, symplostatin-4, myricetin, and fisetin were better than stenopalustroside at trapping FP-2 in its inactive state as revealed by comparative RSMD plots with X-ray structures of FP-2 co-crystallized with inhibitors. Comparative estimates of free energy of binding using the Molecular Mechanics-Poisson Boltzmann Surface Area (MMPBSA) method suggested that His174 protonation may further enhance stenopalustroside–FP-2 interaction. The unique binding signatures of the ligands within the FP-2 active site groove and its sub-sites may explain the subtle differences in their IC50 values and their mechanism of inhibition.










Similar content being viewed by others
References
International Artemisinin Study Group (2004) Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9–17
World Health Organization (2006) Guidelines for the treatment of malaria, 1st edn. World Health Organization, Geneva
Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH et al (2013) Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS ONE 8(3):e57689
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D et al (2008) Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620
Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW (2004) The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis 4(6):327–336
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC et al (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505(7481):50–55
Travassos MA, Laufer MK (2009) Resistance to antimalarial drugs: molecular, pharmacologic, and clinical considerations. Pediatr Res 65(5 Pt 2):64R–70R
Lee SJ, Seo E, Cho Y (2013) Proposal for a new therapy for drug-resistant malaria using plasmodium synthetic lethality inference. Int J Parasitol Drugs Drug Resist 3:119–128
Murphy SC, Harrison T, Hamm HE, Lomasney JW, Mohandas N, Haldar K (2006) Erythrocyte G protein as a novel target for malarial chemotherapy. PLoS Med 3(12):e528
Gardiner DL, Skinner-Adams TS, Brown CL, Andrews KT, Stack CM, McCarthy JS, Dalton JP, Trenholme KR (2009) Plasmodium falciparum: new molecular targets with potential for antimalarial drug development. Expert Rev Anti-Infect Ther 7(9):1087–1098
Lucet IS, Tobin A, Drewry D, Wilks AF, Doerig C (2012) Plasmodium kinases as targets for new-generation antimalarials. Future Med Chem 4(18):2295–2310
Jin H, Xu Z, Cui K, Zhang T, Lu W, Huang J (2014) Dietary flavonoids fisetin and myricetin: dual inhibitors of Plasmodium falciparum falcipain-2 and plasmepsin II. Fitoterapia 94:55–61
Jani D, Nagarkatti R, Beatty W, Angel R, Slebodnick C et al (2008) HDP—A novel heme detoxification protein from the malaria parasite. PLoS Pathog 4(4):e1000053
Chugh M, Sundararaman V, Kumar S, Reddy VS, Siddiqui WA, Stuart KD, Malhotra P (2013) Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proc Natl Acad Sci U S A 110(14):5392–5397
Pandey KC, Barkan DT, Sali A, Rosenthal PJ (2009) Regulatory elements within the prodomain of falcipain-2, a cysteine protease of the malaria parasite Plasmodium falciparum. PLoS ONE 4(5):e5694
Pandey KC, Wang SX, Sijwali PS, Lau AL, McKerrow JH, Rosenthal PJ (2005) The Plasmodium falciparum cysteine protease falcipain-2 captures its substrate, hemoglobin, via a unique motif. Proc Natl Acad Sci U S A 102(26):9138–9143
Soni S, Dhawan S, Rosen KM, Chafel M, Chishti AH, Hanspal M (2005) Characterization of events preceding the release of malaria parasite from the host red blood cell. Blood Cells Mol Dis 35(2):201–211
Domínguez JN, León C, Rodrigues J, Gamboa de Domínguez N, Gut J, Rosenthal PJ (2005) Synthesis and evaluation of new antimalarial phenylurenyl chalcone derivatives. J Med Chem 48:3654–3658
Marco M, Coterón JM (2012) Falcipain inhibition as a promising antimalarial target. Curr Top Med Chem 12(5):408–444
Ettari R, Bova F, Zappalà M, Grasso S, Micale N (2010) Falcipain-2 inhibitors. Med Res Rev 30(1):136–167
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74(4):418–425
Lehane AM, Saliba KJ (2008) Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes 1:26
Hoorn RA, Overkleeft HS, Bogyo M, Kaiser M et al (2012) The antimalarial natural product symplostatin 4 is a nanomolar inhibitor of the food vacuole falcipains. Chem Biol 19(12):1546–1555
Linington RG, Clark BR, Trimble EE, Almanza A, Ureña LD, Kyle DE, Gerwick WH (2009) Antimalarial peptides from marine cyanobacteria: isolation and structural elucidation of gallinamide A. J Nat Prod 72(1):14–17
Hogg T, Nagarajan K, Herzberg S, Chen L, Shen X, Jiang H, Wecke M, Blohmke C, Hilgenfeld R, Schmidt CL (2006) Structural and functional characterization of falcipain-2, a hemoglobinase from the malarial parasite Plasmodium falciparum. J Biol Chem 281(35):25425–25437
Lee HS, Zhang Y (2012) BSP-SLIM: a blind low-resolution ligand-protein docking approach using predicted protein structures. Proteins 80(1):93–110
Molecular Operating Environment (MOE), 2012.10; Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 201
Dyer KM, Perkyns JS, Stell G, Pettitt BM (2009) Site-renormalized molecular fluid theory: on the utility of a two-site model of water. Mol Phys 107:423–431
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718
Schuler LD, Daura X, van Gunsteren WF (2001) An improved GROMOS96 force field for aliphatic hydrocarbons in the condensed phase. J Comput Chem 22(11):1205–1218
Skeel RD (1993) Variable step size destabilizes the Stömer/leapfrog/Verlet method. BIT Numer Math 33:172–175
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Nosé S (1984) A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 52:255–268
Lennard-Jones JE (1924) On the determination of molecular fields. Proc R Soc Lond A 106(738):463–477
Darden T, Perera L, Li L, Pedersen L (1999) New tricks for modelers from the crystallography toolkit: the particle mesh Ewald algorithm and its use in nucleic acid simulations. Structure 7:R55–R60
Páll S, Hess B (2013) A flexible algorithm for calculating pair interactions on SIMD architectures. Comput Phys Commun 184:2641–2650
Fogolari F, Brigo A, Molinari H (2003) Protocol for MM/PBSA molecular dynamics simulations of proteins. Biophys J 85(1):159–166
Kumari R, Kumar R, Open Source Drug Discovery Consortium, Lynn A (2014) g_mmpbsa-A GROMACS Tool for High-Throughput MM-PBSA Calculations. J Chem Inf Model. 2014. [Epub ahead of print]
Wolfram Research, Inc. (2013) MATHEMATICA, Version 9.0, Champaign, IL
The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC
Sinnokrot MO, Sherrill CD (2006) High-accuracy quantum mechanical studies of pi–pi interactions in benzene dimers. J Phys Chem A 110(37):10656–10668
Ringer AL, Sinnokrot MO, Lively RP, Sherrill CD (2006) The effect of multiple substituents on sandwich and T-shaped pi–pi interactions. Chem Eur J 12(14):38218
Buller AR, Townsend CA (2013) Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad. Proc Natl Acad Sci U S A 110(8):E653–E661
Krishtalik LI (2000) The mechanism of the proton transfer: an outline. Biochim Biophys Acta 1458(1):6–27
Quesne MG, Ward RA, de Visser SP (2013) Cysteine protease inhibition by nitrile-based inhibitors: a computational study. Front Chem 1:39
Brinen LS (2009) Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. J Med Chem 52(3):852–857
Omotuyi O, Hamada T (2014) Dynamical footprint of falcipain-2 catalytic triad in hemoglobin-β bound state. J Biomol Struct Dyn 19:1–10
Shah F, Gut J, Legac J, Shivakumar D, Sherman W, Rosenthal PJ, Avery MA (2012) Computer-aided drug design of falcipain inhibitors: virtual screening, structure-activity relationships, hydration site thermodynamics, and reactivity analysis. J Chem Inf Model 52(3):696–710
Shah F, Mukherjee P, Gut J, Legac J, Rosenthal PJ, Tekwani BL, Avery MA (2011) Identification of novel malarial cysteine protease inhibitors using structure-based virtual screening of a focused cysteine protease inhibitor library. J Chem Inf Model 51(4):852–864
Sundararaj S, Singh D, Saxena AK, Vashisht K, Sijwali PS, Dixit R, Pandey KC (2012) The ionic and hydrophobic interactions are required for the auto activation of cysteine proteases of Plasmodium falciparum. PLoS ONE 7(10):e47227
Kerr ID, Lee JH, Pandey KC, Harrison A, Sajid M, Rosenthal PJ, Brinen LS (2009) Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. J Med Chem 52(3):852–857
Hansen G, Heitmann A, Witt T, Li H, Jiang H, Shen X, Heussler VT, Rennenberg A, Hilgenfeld R (2011) Structural basis for the regulation of cysteine-protease activity by a new class of protease inhibitors in plasmodium. Structure 19(7):919–929
Wang SX, Pandey KC, Scharfstein J, Whisstock J, Huang RK, Jacobelli J, Fletterick RJ, Rosenthal PJ, Abrahamson M, Brinen LS, Rossi A, Sali A, McKerrow JH (2007) The structure of chagasin in complex with a cysteine protease clarifies the binding mode and evolution of an inhibitor family. Structure 15(5):535–543
Zhang W, Huang J, Shan L, Li H, Wang L, Zhang S, Lu W, Su J, Chen TA (2012) method for treatment of malaria including administering to a patient in need thereof a flavonoid glycoside compound. US Patent Application No. US 2012/0295859 A1
Conroy T, Guo JT, Hunt NH, Payne RJ (2010) Total synthesis and antimalarial activity of symplostatin 4. Org Lett 12(23):5576–5579
Conroy T, Guo JT, Linington RG, Hunt NH, Payne RJ (2011) Total synthesis, stereochemical assignment, and antimalarial activity of gallinamide A. Chemistry 17(48):13544–13552
Omotuyi IO, Oluyemi KA, Omofoma CO, Josiah SJ, Adesanya OA, Saalu LC (2006) Cyfluthrin induced hepatotoxicity in rats. Afr J Biotechnol 5(20):1909–1912
Schleier JJ, Peterson RK (2012) The joint toxicity of type I, II, and nonester pyrethroid insecticides. J Econ Entomol 105(1):85–91
Omotuyi IO, Nwangwu SC, Okugbo OT, Okoye OT, Ojieh GC, Wogu DM (2008) Hepatotoxic and hemolytic effects of acute exposure of rats to artesunate overdose. Afr J Biochem Res 2:107–110
Efferth T, Kaina B (2010) Toxicity of the antimalarial artemisinin and its derivatives. Crit Rev Toxicol 40(5):405–421
Bero J, Frédérich M, Quetin-Leclercq J (2009) Antimalarial compounds isolated from plants used in traditional medicine. J Pharm Pharmacol 61(11):1401–1433
Groeger AL, Freeman BA (2010) Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Mol Interv 10(1):39–50
Zhang WH, Liu J, Xu G, Yuan Q, Sayre LM (2003) Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem Res Toxicol 16:512–523
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDB 303 kb)
Rights and permissions
About this article
Cite this article
Omotuyi, O.I. Methyl-methoxylpyrrolinone and flavinium nucleus binding signatures on falcipain-2 active site. J Mol Model 20, 2386 (2014). https://doi.org/10.1007/s00894-014-2386-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2386-2