Abstract
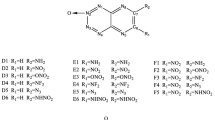
s-heptazine is one of the most attractive polycyclic C-N precursors for graphitic carbon nitride materials (CNx). In this paper in order to find the relationships between the structure, aromaticity, and stability for this novel compound, its analogues with three conjoint six-membered rings (I ∼ V) and derivatives with different substituents (VI-1 ∼ VI-5) were investigated using the density functional theory method. Aromaticity was predicted using the magnetic criterion iso-chemical shielding surface in the Z direction (ICSSzz) obtained with the gauge-independent atomic orbital (GIAO) method. Stability was estimated by the band gap and the topological properties obtained from the atoms in molecules theory. Results show that replacement of the CH groups with the nitrogen atoms in the tricyclic core enhances both the aromaticity and the stability. s-heptazine (VI) that has the maximum number of N atoms among analogues I ∼ VI possesses the largest aromaticity and the best stability. Substitutions of -NH2, −NHNH2, and -N3 groups increase not only the aromaticity but also the stability; −NO2 increases the aromaticity while decreases the stability; −CN decreases both the aromaticity and the stability. Furthermore, the energetic performance of VI-1 ∼ VI-5 was evaluated according to the estimated specific impulse (Is). The obtained Is has the order of VI-5 > VI-4 > VI-3 > VI > VI-1 > VI-2. The Is of VI-5 is higher than that of HMX (1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetraazacyclooctane).








Similar content being viewed by others
References
Goglio G, Foy D, Demazeau G (2008) State of art and recent trends in bulk carbon nitrides synthesis. Materials Science and Engineering 58:195–227
Shahbaz M, Urano S, LeBreton PR, Rossman MA, Hosmane RS, Leonard NJ (1984) Tri-s-triazine: synthesis, chemical behavior, and spectroscopic and theoretical probes of valence orbital structure. Journal of the American Chemical Society 106:2805–2811
Hosmane RS, Rossman MA, Leonard NJ (1982) Synthesis and structure of tri-s-triazine. Journal of the American Chemical Society 104:5497–5499
Kroke E, Schwarz M, Horath-Bordon E, Kroll P, Noll B, Norman AD (2002) Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New Journal of Chemistry 26:508–512
Jürgens B, Irran E, Senker J, Kroll P, Müller H, Schnick W (2003) Melem (2, 5, 8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. Journal of the American Chemical Society 125:10288–10300
Miller DR, Swenson DC, Gillan EG (2004) Synthesis and structure of 2, 5, 8-triazido-s-heptazine: an energetic and luminescent precursor to nitrogen-rich carbon nitrides. Journal of the American Chemical Society 126:5372–5373
Saplinova T, Bakumov V, Gmeiner T, Wagler J, Schwarz M, Kroke E (2009) 2, 5, 8-Trihydrazino-s-heptazine: a precursor for heptazine-based iminophosphoranes. Zeitschrift für Anorganische und Allgemeine Chemie 635:2480–2487
Giacomelli G, Porcheddu A (2008) In :Katritzky AR, Christopher A, Scriven, EFV, Taylor, RK, (eds) 1,3,5-Triazines. Elsevier, Oxford
Holst JR, Gillan EG (2008) From triazines to heptazines: deciphering the local structure of amorphous nitrogen-rich carbon nitride materials. Journal of the American Chemical Society 130:7373–7379
Krygowski TM (1993) Crystallographic studies of inter-and intramolecular interactions reflected in aromatic character of π -electron systems. Journal of Chemical Information and Computer Sciences 33:70–78
Kalinowska M, Siemieniuk E, Kostro A, Lewandowski W (2006) The application of Aj, BAC, I6, HOMA indexes for quantitative determination of aromaticity of metal complexes with benzoic, salicylic, nicotinic acids and benzene derivatives. Journal of Molecular Structure: THEOCHEM 761:129–141
Noorizadeh S, Shakerzadeh E (2010) Shannon entropy as a new measure of aromaticity Shannon aromaticity. Physical Chemistry Chemical Physics 12:4742–4749
Noorizadeh S, Shakerzadeh E (2011) Aromaticity study on tria-, penta-and hepta-fulvene derivatives. Computational and Theoretical Chemistry 964:141–147
Mayer I (2012) Improved definition of bond orders for correlated wave functions. Chemical Physics Letters 544:83–86
Glukhovtsev MN, von Ragué SP (1992) Structures, bonding and energies of N6 isomers. Chemical Physics Letters 198:547–554
Suresh CH, Koga N (2002) Accurate calculation of aromaticity of benzene and antiaromaticity of cyclobutadiene: new homodesmotic reactions. The Journal of Organic Chemistry 67:1965–1968
Schleyer PR (1996) Maerker C, Dransfeld A, Jiao H, Hommes NJvE, Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. Journal of the American Chemical Society 118:6317–6318
Bader RF, Atoms in Molecules. International Series of Monographs in Chemistry. 1990, Oxford: Oxford University Press.
Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R (2006) Schleyer PvR, Which NICS aromaticity index for planar π rings is best? Organic Letters 8:863–866
Corminboeuf C, Heine T, Seifert G, von Ragué SP, Weber J (2004) Induced magnetic fields in aromatic [n]-annulenes—interpretation of NICS tensor components. Physical Chemistry Chemical Physics 6:273–276
Heine T, von Ragué Schleyer P, Corminboeuf C, Seifert G, Reviakine R, Weber J (2003) Analysis of aromatic delocalization: individual molecular orbital contributions to nucleus-independent chemical shifts. The Journal of Physical Chemistry A 107:6470–6475
Lu T, Multiwfn, version 3.2.1, http://multiwfn.codeplex.com/, 2013.
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. Journal of Chemical Physics 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B 37:785–789
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theoretica Chimica Acta 28:213–222
Frisch MJ TG, Schlegel HB, Suzerain GE, Robb MA, Cheeseman JrJR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision B.05. Gaussian Inc, Pittsburgh, PA
Cheeseman JR, Trucks GW, Keith TA, Frisch MJ (1996) A comparison of models for calculating nuclear magnetic resonance shielding tensors. The Journal of Chemical Physics 104:5497–5509
Schreckenbach G, Ziegler T (1995) Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. The Journal of Physical Chemistry 99:606–611
Politzer P, Murray J, Grice M, Sjoberg P, Olah G, Squire D (1991) Chemistry of energetic materials. Academic, San Diego
Yang JQ, Wang F, Zhang JY, Wang GX, Gong XD (2013) A theoretical study on 1, 5-diazido-3-nitrazapentane (DANP) and 1, 7-diazido-2, 4, 6-trinitrazaheptane (DATNH): molecular and crystal structures, thermodynamic and detonation properties, and pyrolysis mechanism. Journal of Molecular Modeling 19:5367–5376
Yang JQ, Yan H, Zhang XL, Wang GX, Gong XD (2014) A theoretical prediction of the molecular and electronic structures, thermodynamic properties, and stability of 1, 3-diazido-2-methyl-2-nitropropane (DAMNP). Structural Chemistry 25:931–940
Keßenich E, Klapötke TM, Knizek J, Nöth H, Schulz A (1998) Characterization, crystal structure of 2, 4-bis (triphenylphosphanimino) tetrazolo [5, 1-a]-[1, 3, 5] triazine, and improved crystal structure of 2, 4, 6-Triazido-1, 3, 5-triazine. European Journal of Inorganic Chemistry 1998:2013–2016
Yang JQ, Yan H, Wang GX, Zhang XL, Wang TY, Gong XD (2014) Computational investigations into the substituent effects of –N3, −NF2, −NO2, and –NH2 on the structure, sensitivity and detonation properties of N, N’-azobis (1, 2, 4-triazole). Journal of Molecular Modeling 20: DOI: 10.1007/s00894-00014-02148-00891
Mezey PG (1993) Shape in chemistry: an introduction to molecular shape and topology. VCH, New York.
Zheng W, Wong N-B, Li W-K, Tian A (2004) Tri-s-triazine and its nitrogen isoelectronic equivalents: an ab initio study. The Journal of Physical Chemistry A 108:11721–11727
Ju X-H, Wang Z-Y, Yan X-F, Xiao H-M (2007) Density functional theory studies on dioxygen difluoride and other fluorine/oxygen binary compounds: availability and shortcoming. Journal of Molecular Structure: THEOCHEM 804:95–100
Wang F, Du H, Zhang J, Gong X (2011) Computational studies on the crystal structure, thermodynamic properties, detonation performance, and pyrolysis mechanism of 2, 4, 6, 8-tetranitro-1, 3, 5, 7-tetraazacubane as a novel high energy density material. The Journal of Physical Chemistry A 115:11788–11795
Bliss DE, Christian SL, Wilson WS (1991) Impact sensitivity of polynitroaromatics. Journal of Energetic Materials 9:319–348
von Ragué SP, Manoharan M, Wang Z-X, Kiran B, Jiao H, Puchta R, van Eikema Hommes NJ (2001) Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Organic Letters 3:2465–2468
Minkin VI, Glukhovtsev MN, Simkin B (1994) Aromaticity and antiaromaticity. Wiley, New York
Garratt PJ (1986) Aromaticity. Wiley, New York
Liu YF, An HM, Yang RJ (2001) Combustion properties of nitroamine monopropellants. Chinese Journal of Explosives & Propellants 24:48–49
Acknowledgments
We gratefully thank the Research Fund for Natural Science Foundation of Jiangsu Province (NO.BK20130755) and the “Excellent Plan and Zijin Star” Research Foundation of National University of Science and Technology for their support to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Gong, X. & Wang, G. Structure, aromaticity, stability, and energetic performance of the analogues and derivatives of s-heptazine. J Mol Model 20, 2379 (2014). https://doi.org/10.1007/s00894-014-2379-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2379-1