Abstract
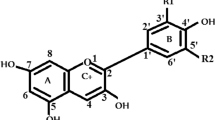
UV-Vis spectra were calculated using time-dependent density functional theory for the chrysanthemin pigment, which is used as natural dye in dye sensitized solar cells. To this end, we studied four different calculation protocols in order to obtain the best approximation according to the maximum absorption wavelength (λmax) of the experimental spectrum. Furthermore, the optimized geometry, highest occupied molecular orbitals, lowest unoccupied molecular orbitals and electron density were calculated and analyzed. Several chemical models were used with and without the presence of the chlorine atom: the chosen functionals, B3LYP, PBE0 and the M06 family, represent various approximations with different fractions of Hartree-Fock exchange energy. These functionals were combined with the 6–31 + G (d), 6–311 + G (d) and the MIDIX + basis sets. All of these calculation protocols proved a good option, though the B3LYP/MIDIX + chemistry model was the best for predicting the λmax value, using the equilibrium calculation protocol (M1a) in the presence of chlorine.






Similar content being viewed by others
References
Valls J, Millán S, Martí MP, Borràs E, Arola L (2009) Advanced separation methods of food anthocyanins, isoflavones and flavanols. J Chromatogr A 1216(43):7143–7172. doi:10.1016/j.chroma.2009.07.030
Calogero G, Marco GD (2008) Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol Energy Mater Sol Cells 92(11):1341–1346. doi:10.1016/j.solmat.2008.05.007
Bechtold T, Mussak R (2009) Handbook of natural colorants. Wiley, Chichester
Polo AS, Murakami Iha NY (2006) Blue sensitizers for solar cells: natural dyes from calafate and jaboticaba. Sol Energy Mater Sol Cells 90(13):1936–1944. doi:10.1016/j.solmat.2006.02.006
Chang H, Lo Y-J (2010) Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol Energy 84(10):1833–1837. doi:10.1016/j.solener.2010.07.009
Wongcharee K, Meeyoo V, Chavadej S (2007) Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol Energy Mater Sol Cells 91(7):566–571. doi:10.1016/j.solmat.2006.11.005
Senthil TS, Muthukumarasamy N, Velauthapillai D, Agilan S, Thambidurai M, Balasundaraprabhu R (2011) Natural dye (cyanidin 3-O-glucoside) sensitized nanocrystalline TiO2 solar cell fabricated using liquid electrolyte/quasi-solid-state polymer electrolyte. Renew Energy 36(9):2484–2488. doi:10.1016/j.renene.2011.01.031
Calogero G, Yum J-H, Sinopoli A, Di Marco G, Grätzel M, Nazeeruddin MK (2012) Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol Energy 86(5):1563–1575. doi:10.1016/j.solener.2012.02.018
O'Regan BG, Michael (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353 (6346):4. doi: 10.1038/353737a0
Grätzel M (2003) Dye-sensitized solar cells. J Photochem Photobiol C: Photochem Rev 4(2):145–153. doi:10.1016/S1389-5567(03)00026-1
Narayan MR (2012) Review: Dye sensitized solar cells based on natural photosensitizers. Renew Sust Energ Rev 16(1):208–215. doi:10.1016/j.rser.2011.07.148
Nazeeruddin MK, Kay A, Rodicio I, Humphry-Baker R, Mueller E, Liska P, Vlachopoulos N, Graetzel M (1993) Conversion of light to electricity by cis-X2bis (2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium (II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115(14):6382–6390. doi:10.1021/ja00067a063
Nazeeruddin K, Pechy P, Gratzel M (1997) Efficient panchromatic sensitization of nanocrystalline TiO2 films by a black dye based on a trithiocyanato-ruthenium complex. Chem Commun 18:1705–1706. doi:10.1039/A703277C
Nazeeruddin MK, De Angelis F, Fantacci S, Selloni A, Viscardi G, Liska P, Ito S, Takeru B, Grätzel M (2005) Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J Am Chem Soc 127(48):16835–16847. doi:10.1021/ja052467l
Grätzel M (2009) Recent advances in sensitized mesoscopic solar cells. Accounts Chem Res 42(11):1788–1798. doi:10.1021/ar900141y
Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H (2003) Molecular design of coumarin dyes for efficient dye-sensitized solar cells. J Phys Chem B 107(2):597–606. doi:10.1021/jp026963x
Hara K, Sato T, Katoh R, Furube A, Yoshihara T, Murai M, Kurashige M, Ito S, Shinpo A, Suga S, Arakawa H (2005) Novel conjugated organic dyes for efficient dye-sensitized solar cells. Adv Funct Mater 15(2):246–252. doi:10.1002/adfm.200400272
Hagberg DP, Marinado T, Karlsson KM, Nonomura K, Qin P, Boschloo G, Brinck T, Hagfeldt A, Sun L (2007) Tuning the HOMO and LUMO energy levels of organic chromophores for dye sensitized solar cells. J Org Chem 72(25):9550–9556. doi:10.1021/jo701592x
Hwang S, Lee JH, Park C, Lee H, Kim C, Park C, Lee M-H, Lee W, Park J, Kim K, Park N-G, Kim C (2007) A highly efficient organic sensitizer for dye-sensitized solar cells. Chem Commun 46:4887–4889. doi:10.1039/B709859F
Zeng W, Cao Y, Bai Y, Wang Y, Shi Y, Zhang M, Wang F, Pan C, Wang P (2010) Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chem Mater 22(5):1915–1925. doi:10.1021/cm9036988
Sandquist C, McHale JL (2011) Improved efficiency of betanin-based dye-sensitized solar cells. J Photochem Photobiol A Chem 221(1):90–97. doi:10.1016/j.jphotochem.2011.04.030
Cossi M, Barone V (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115(10):4708–4717. doi:10.1063/1.1394921
Improta R, Barone V, Scalmani G, Frisch MJ (2006) A state-specific polarizable continuum model time dependent density functional theory method for excited state calculations in solution. J Chem Phys 125 (5):054103. doi: 10.1063/1.2222364
Dai Q, Rabani J (2002) Photosensitization of nanocrystalline TiO2 films by anthocyanin dyes. J Photochem Photobiol A Chem 148(1–3):17–24. doi:10.1016/S1010-6030(02)00073-4
Barone V, Ferretti A, Pino I (2012) Absorption spectra of natural pigments as sensitizers in solar cells by TD-DFT and MRPT2: protonated cyanidin. Phys Chem Chem Phys 14(46):16130–16137. doi:10.1039/C2CP42657A
Becke AD (1993) Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. doi:10.1063/1.464913
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170. doi:10.1063/1.478522
Dev P, Agrawal S, English NJ (2012) Determining the appropriate exchange-correlation functional for time-dependent density functional theory studies of charge-transfer excitations in organic dyes. J Chem Phys 136(22):224301. doi: 10.1063/1.4725540
Ekanayake P, Kooh MRR, Kumara NTRN, Lim A, Petra MI, Voo NY, Lim CM (2013) Combined experimental and DFT–TDDFT study of photo-active constituents of Canarium odontophyllum for DSSC application. Chem Phys Lett 585:121–127. doi:10.1016/j.cplett.2013.08.094
Mohammadi N, Mahon PJ, Wang F (2013) Toward rational design of organic dye sensitized solar cells (DSSCs): an application to the TA-St-CA dye. J Mol Graph Model 40:64–71. doi:10.1016/j.jmgm.2012.12.005
Preat J, Jacquemin D, Wathelet V, André J-M, Perpète EA (2007) Towards the understanding of the chromatic behaviour of triphenylmethane derivatives. Chem Phys 335(2–3):177–186. doi:10.1016/j.chemphys.2007.04.014
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125 (19):194101. doi: 10.1063/1.2370993
Zhao Y, Truhlar D (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120(1–3):215–241. doi:10.1007/s00214-007-0310-x
Zhao Y, Truhlar DG (2006) Density Functional for Spectroscopy: No Long-Range Self-Interaction Error, Good Performance for Rydberg and Charge-Transfer States, and Better Performance on Average than B3LYP for Ground States. J Phys Chem A 110(49):13126–13130. doi:10.1021/jp066479k
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA (2001) 6-31G* basis set for third-row atoms. J Comput Chem 22(9):976–984. doi:10.1002/jcc.1058
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self‐consistent molecular orbital methods. XXIII. A polarization‐type basis set for second‐row elements. J Chem Phys 77(7):3654–3665. doi:10.1063/1.444267
Lynch BJ, Truhlar DG (2004) Small basis sets for calculations of barrier heights, energies of reaction, electron affinities, geometries, and dipole moments. Theor Chem Account 111(2–6):335–344. doi:10.1007/s00214-003-0518-3
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72(1):650–654. doi:10.1063/1.438955
Gaussian 09, Revision A.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov A F, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J W, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Sanchez-de-Armas R, San Miguel MA, Oviedo J, Sanz JF (2012) Coumarin derivatives for dye sensitized solar cells: a TD-DFT study. Phys Chem Chem Phys 14(1):225–233. doi:10.1039/C1CP22058F
Sánchez-de-Armas R, San-Miguel MA, Oviedo J, Sanz JF (2012) Molecular modification of coumarin dyes for more efficient dye sensitized solar cells. J Chem Phys 136 (19):194702. doi: 10.1063/1.4711049
Zhang J, Kan Y-H, Li H-B, Geng Y, Wu Y, Su Z-M (2012) How to design proper π-spacer order of the D-π-A dyes for DSSCs? A density functional response. Dyes Pigments 95(2):313–321. doi:10.1016/j.dyepig.2012.05.020
Lu X, Wei S, Wu C-ML, Li S, Guo W (2011) Can polypyridyl Cu (I)-based complexes provide promising sensitizers for dye-sensitized solar cells? A theoretical insight into Cu (I) versus Ru (II) sensitizers. J Phys Chem C 115(9):3753–3761. doi:10.1021/jp111325y
Muscat J, Wander A, Harrison NM (2001) On the prediction of band gaps from hybrid functional theory. Chem Phys Lett 342(3–4):397–401. doi:10.1016/S0009-2614(01)00616-9
Zhao Y, Truhlar DG (2009) Calculation of semiconductor band gaps with the M06-L density functional. J Chem Phys 130 (7):074103. doi: 10.1063/1.3076922
Xiao H, Tahir-Kheli J, Goddard WA (2011) Accurate band gaps for semiconductors from density functional theory. J Phys Chem Lett 2(3):212–217. doi:10.1021/jz101565j
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) and Centro de Investigación en Materiales Avanzados, S.C. (CIMAV), and Universidad Autónoma de Sinaloa. R.S.R. and J.B.L. gratefully acknowledge a fellowship from CONACYT. D.G.M and N.F.H. are researchers of CIMAV and CONACYT.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.35 MB)
Rights and permissions
About this article
Cite this article
Soto-Rojo, R., Baldenebro-López, J., Flores-Holguín, N. et al. Comparison of several protocols for the computational prediction of the maximum absorption wavelength of chrysanthemin. J Mol Model 20, 2378 (2014). https://doi.org/10.1007/s00894-014-2378-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2378-2