Abstract
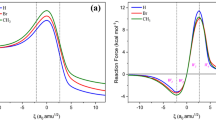
The course of the Diels-Alder reactions of cyclopentadiene and maleic anhydride were studied. Two reaction paths were modelled: endo- and exo-selective paths. All structures within the transient region were characterized and analyzed by means of geometrical descriptors, physicochemical parameters and information-theoretical measures in order to observe the linkage between chemical behavior and the carriage of information. We have shown that the information-theoretical characterization of the chemical course of the reaction is in complete agreement with its phenomenological behavior in passing from reactants to products. In addition, we were able to detect the main differences between the two reaction mechanisms. This type of informational analysis serves to provide tools to help understand the chemical reactivity of the two simplest Diels-Alder reactions, which permits the establishment of a connection between the quantum changes that molecular systems exert along reaction coordinates and standard physicochemical phenomenology. In the present study, we have shown that every reaction stage has a family of subsequent structures that are characterized not solely by their phenomenological behavior but also by informational properties of their electronic density distribution (localizability, order, uniformity). Moreover, we were able to describe the main differences between endo-adduct and exo-adduct pathways. With the advent of new experimental techniques, it is in principle possible to observe the structural changes in the transient regions of chemical reactions. Indeed, through this work we have provided the theoretical concepts needed to unveil the concurrent processes associated with chemical reactions.













Similar content being viewed by others
References
Eyring H (1935) The activated complex in chemical reactions. J Chem Phys 3:107
Wigner E (1938) The transition state method. Trans Faraday Soc 34:29–41
Fukui K (1981) Acc Chem Res 14:363–368
Shaik S, Ioffe A, Reddy AC, Pross A (1994) J Am Chem Soc 116:213–262
Polanyi JC, Zewail AH (1995) Direct observation of the transition state. Accs Chem Research 28(3):119–132
Zewail AH (2000) Femtochemistry: atomic-scale dynamics of the chemical bond using ultrafast lasers (Nobel Lecture). Angewandte Chem Int Ed 39(15):2586–2631
Politzer P, Toro-Labbé A, Gutiérrez-Oliva S, Herrera B, Jaque P, Concha MC et al (2005) The reaction force: three key points along an intrinsic reaction coordinate. J Chem Sci 117(5):467–472
Toro-Labbé A, Gutiérrez-Oliva S, Murray J, Politzer P (2007) A new perspective on chemical and physical processes: the reaction force. Mol Phys 105:2619–2625
Shi Z, Boyd RJ (1991) J Am Chem Soc 113:1072–1076
Bader RFW, MacDougall PJ (1985) J Am Chem Soc 107:6788–6795
Knoerr EH, Eberhart ME (2001) J Phys Chem A 105:880–884
Shaik SS, Schlegel HB, Wolfe S (1992) Theoretical aspects of physical organic chemistry: the SN2 reaction. Wiley, New York
Tachibana AJ (2001) Chem Phys 115:3497–3518
Borgoo A, Jaque P (2009) Toro-Labbe´ A, Van Alsenoy C, Geerlings P. Phys Chem Chem Phys 11:476–482
Rong C, Lu T, Liu S (2014) Dissecting molecular descriptors into atomic contributions in density functional reactivity theory. J Chem Phys 140(2):024109
Gadre SR (1984) Information entropy and Thomas-Fermi theory. Phys Rev A 30:620–621; Gadre SR, Bendale RD, Gejji SP (1985) Analysis of atomic electron momentum densities: Use of information entropies in coordinate and momentum space. Chem Phys Lett 117:138–142; Gadre SR, Bendale RD (1985) Information entropies in quantum chemistry. Curr Sci 54:970–977; Gadre SR, Sears SB, Chakravorty SJ, Bendale RD (1985) Some novel characteristics of atomic information entropies. Phys Rev A 32:2602–2606; Koga T, Morita M (1983) Maximum-entropy inference and momentum density approach. J Chem Phys 79:1933–1938; Ghosh SK, Berkowitz M, Parr, RG (1984) Transcription of ground-state density-functional theory into a local thermodynamics. Proc Natl Acad Sci USA 81:8028–8031; Angulo JC, Dehesa JS (1992) Tight rigorous bounds to atomic information entropies. J Chem Phys 97:6485–6495; Massen SE, Panos CP (1998) Universal property of the information entropy in atoms, nuclei and atomic clusters. Phys Lett A 246:530–533; Nalewajski RF, Parr RG (2001) Information theory thermodynamics of molecules and their Hirshfeld fragments. J Phys Chem A 105:7391–7400; Nagy A (2003) Fisher information in density functional theory. J Chem Phys 119:9401–9405; Romera E, Dehesa JS (2004) The Fisher–Shannon information plane, an electron correlation tool. J Chem Phys 120:8906–8917; Karafiloglou P, Panos C (2004) Order of Coulomb and Fermi pairs: Application in a π-system. Chem Phys Lett 389:400–404; Sen KD (2005) Characteristic features of Shannon information entropy of confined atoms. J Chem Phys 123:074110; Parr RG, Ayers PW, Nalewajski RF (2005) What is an atom in a molecule? J Phys Chem A 109:3957–3959; González-Férez R, Dehesa JS (2005) Characterization of atomic avoided crossings by means of Fisher’s information. Eur Phys J D 32:39–43; Guevara NL, Sagar RP, Esquivel RO (2005) Local correlation measures in atomic systems. J Chem Phys 122:084101; Shi Q, Kais S (2005) Discontinuity of Shannon information entropy for two-electron atoms. Chem Phys 309:127–131; Chatzisavvas KC, Moustakidis CC, Panos CP (2005) Information entropy, information distances, and complexity in atoms. J Chem Phys 123:174111; Sen KD, Katriel J (2006) Information entropies for eigen densities of homogeneous potentials. J Chem Phys 125:074117; Nagy A (2006) Fisher information in a two-electron entangled artificial atom. Chem Phys Lett 425:154–156; Ayers PW (2006) Density bifunctional theory using the mass density and the charge density. Theor Chem Acc 115:253–256; Martyushev LM, Seleznev VD (2006) Maximum entropy production principle in physics, chemistry and biology. Phys Rep 426:1–45; Liu S (2007) On the relationship between densities of Shannon entropy and Fisher information for atoms and molecules. J Chem Phys 126:191107; Geerlings P, Borgoo A (2011) Information carriers and (reading them through) information theory in quantum chemistry. Phys Chem Chem Phys 13:911–922
Esquivel RO, Angulo JC, Dehesa JS, Antolín J, López-Rosa S, Flores-Gallegos N, Molina-Espíritu M, Iuga C (2012) Recent advances toward the nascent science of quantum information chemistry. In: Deloumeaux P, Gorzalka JD (eds) Information theory: new research. Nova Science, Hauppauge, NY
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793
Gaussian 09, Revision C.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian, Inc., Wallingford CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Becke AD (1993) Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Pérez-Jordá JM, Becke AD, San-Fabián E (1994) Automatic numerical integration techniques for polyatomic molecules. J Chem Phys 100:6520
Kohout M (2013) Program DGRID, version 4.6
Todeschini R, Consonni V (2003) Descriptors from molecular geometry. Handbook of Chemoinformatics: From Data to Knowledge in 4 Volumes, 1004–1033
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Academic, New York
Koopmans T (1934) Physica 1:1
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Parr RG, von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922
Pearson RGJ (1963) Am Chem Soc 85:3533; Pearson RG (1966) Science 151:172
Pearson RG Inorg Chem (1988) 27:734; Pearson RGJ (1988) Am Chem Soc 110:7684
Pearson RG (1990) Coord Chem Rev 100:403
Shannon C, Weaver W (2002) Mathematical theory of communication. University of Illinois Press, Chicago, IL
Fisher RA (1925) Theory of statistical estimation. Mathematical Proceedings of the Cambridge Philosophical. Cambridge University Press, Society
36.Frieden BR (2004) Science from Fisher information: a unification. Cambridge University Press
Carbó R, Leyda L, Arnau M (1980) How similar is a molecule to another? An electron density measure of similarity between two molecular structures. Int J Quant Chem 17(6):1185–1189
Onicescu O (1966) Energie informationnelle. CR Acad Sci Paris Ser A 263:841–842
Hamilton I, Mosna RA (2010) Fisher information and kinetic energy functionals: a dequantization approach. J Comp Appl Math 233(6):1542–1547
Esquivel RO, Flores-Gallegos N, Iuga C, Carrera EM, Angulo JC, Antolín J (2009) Phenomenological description of the transition state, and the bond breaking and bond forming processes of selected elementary chemical reactions: an information-theoretic study. Theor Chem Acc 124(5–6):445–460
Esquivel RO, Molina-Espíritu M, Dehesa JS, Angulo JC, Antolín J (2012) Concurrent phenomena at the transition region of selected elementary chemical reactions: an information-theoretical complexity analysis. Int J Quantum Chem 112(22):3578–3586
Molina-Espíritu M, Esquivel RO, Angulo JC, Antolín J, Dehesa JS (2012) Information-theoretical complexity for the hydrogenic identity SN2 exchange reaction. J Math Chem 50(7):1882–1900
Molina-Espíritu M, Esquivel RO, Angulo JC, Antolín J, Iuga C, Dehesa JS (2013) Information‐theoretical analysis for the SN2 exchange reaction CH3Cl + F−. Int J Quantum Chem 113:2589
Acknowledgments
We wish to thank José María Pérez-Jordá for kindly providing his numerical code. R.O.E. wishes to thank Juan Carlos Angulo and Jesús Sánchez-Dehesa for their kind hospitality during his 2012–2014 sabbatical stay at Universidad de Granada, Spain. R.O.E. thanks Consejo Nacional de Ciencia y Tecnología (CONACyT)-México for financial support. M.M.-E. wishes to thank CONACyT for a PhD fellowship. We acknowledge financial support through Mexican grants from CONACyT, Programa Integral de Fortalecimiento Institutcional (PIFI), Programa del Mejoramiento del Profesorado (PROMEP-SEP) and Spanish grants FIS2011-24540, FQM-7276 and FQM-4643. J.C.A. and R.O.E. belong to the Andalusian research group FQM-020 and J.S.D. to FQM-0207. Allocation of supercomputing time from Laboratorio de Supercómputo y Visualización (U.A.M.), Sección de Supercomputación at CSIRC (U. de G.) and from Proteus (Instituto “Carlos I”) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013.
Rights and permissions
About this article
Cite this article
Molina-Espíritu, M., Esquivel, R.O., Kohout, M. et al. Insight into the informational-structure behavior of the Diels-Alder reaction of cyclopentadiene and maleic anhydride. J Mol Model 20, 2361 (2014). https://doi.org/10.1007/s00894-014-2361-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2361-y