Abstract
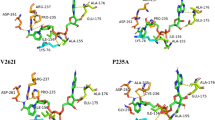
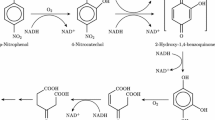
Polycyclic aromatic hydrocarbons are a family of ubiquitous pollutants whose environmental behavior has been widely studied. Different bacterial species are able to decompose hydrocarbons by using them as a food source. One of the best-studied enzymes is naphthalene 1,2-dioxygenase (NDO). A practical way to optimize the degradation process is by mutating the protein involved, increasing both the degradation capacity of the enzyme and its ability to work under extreme environmental conditions of high temperature and low pH. Herein, we describe the study of NDO using molecular dynamics and docking calculations to discover new mutants with high degrading capabilities. We modeled eleven new mutants of NDO. The results indicate that increasing the size of the active site cavity in the mutants allowed for the insertion of high molecular weight PAHs. Additionally, the physicochemical properties of the NDO active sites make the sites well suited to interactions with PAHs, so most amino-acid modifications should not result in significantly altered behavior of NDO.







Similar content being viewed by others
References
Librando V, Fazzino SD (1993) Quantification of polycyclic aromatic-hydrocarbons and their nitro-derivatives in atmospheric particulate matter of Augusta City. Chemosphere 27(9):1649–1656. doi:10.1016/0045-6535(93)90146-V
Castelli F, Librando V, Sarpietro MG (2002) Calorimetric approach of the interaction and absorption of polycyclic aromatic hydrocarbons with model membranes. Environ Sci Technol 36(12):2717–2723. doi:10.1021/Es010260w
Jeffy BD, Chirnomas RB, Romagnolo DF (2002) Epigenetics of breast cancer: polycyclic aromatic hydrocarbons as risk factors. Environ Mol Mutagen 39(2–3):235–244. doi:10.1002/Em.10051
Laupeze W, Amiot L, Sparfel L, Le Ferrec E, Fauchet R, Fardel O (2002) Polycyclic aromatic hydrocarbons affect functional differentiation and maturation of human monocyte-derived dendritic cells. J Immunol 168(6):2652–2658
Hellou J, Leonard J, Anstey C (2002) Dietary exposure of finfish to aromatic contaminants and tissue distribution. Arch Environ Contam Toxicol 42(4):470–476. doi:10.1007/s00244-001-0038-x
Librando V, Perrini G, Tomasello M (2002) Biomonitoring of atmospheric PAHs by evergreen plants: correlations and applicability. Polycycl Aromat Compd 22(3–4):549–559. doi:10.1080/10406630213563
Chen SH, Aitken MD (1999) Salicylate stimulates the degradation of high molecular weight polycyclic aromatic hydrocarbons by Pseudomonas saccharophila P15. Environ Sci Technol 33(3):435–439. doi:10.1021/Es9805730
Simon MJ, Osslund TD, Saunders R, Ensley BD, Suggs S, Harcourt A, Suen WC, Cruden DL, Gibson DT, Zylstra GJ (1993) Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and Ncib-9816-4. Gene 127(1):31–37. doi:10.1016/0378-1119(93)90613-8
Goyal AK, Zylstra GJ (1996) Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl Environ Microbiol 62(1):230–236
Schneider J, Grosser R, Jayasimhulu K, Xue WL, Warshawsky D (1996) Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site (vol 62, pg 14, 1996). Appl Environ Microbiol 62(4):1491–1491
Goyal AK, Zylstra GJ (1997) Genetics of naphthalene and phenanthrene degradation by Comamonas testosteroni. J Ind Microbiol Biotechnol 19(5–6):401–407. doi:10.1038/sj.jim.2900476
Gibson DT, Parales RE (2000) Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol 11(3):236–243. doi:10.1016/S0958-1669(00)00090-2
Resnick SM, Lee K, Gibson DT (1996) Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol Biotechnol 17(5–6):438–457. doi:10.1007/Bf01574775
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3(2–3):17
Yu CL, Parales RE, Gibson DT et al (2001) Multiple mutations at the active site of naphthalene dioxygenase affect regioselectivity and enantioselectivity. J Ind Microbiol Biotechnol 27(2):94–103. doi:10.1038/sj.jim.7000168
Seo J, Kang SI, Kim M, Han J, Hur HG (2011) Flavonoids biotransformation by bacterial non-heme dioxygenases, biphenyl and naphthalene dioxygenase. Appl Microbiol Biotechnol 91(2):219–228. doi:10.1007/s00253-011-3334-z
Seo J, Ryu JY, Han J, Ahn JH, Sadowsky MJ, Hur HG, Chong Y (2013) Amino acid substitutions in naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816–4 result in regio- and stereo-specific hydroxylation of flavanone and isoflavanone. Appl Microbiol Biotechnol 97(2):693–704. doi:10.1007/s00253-012-3962-y
Ferraro DJ, Okerlund AL, Mowers JC, Ramaswamy S (2006) Structural basis for regioselectivity and stereoselectivity of product formation by naphthalene 1,2-dioxygenase. J Bacteriol 188(19):6986–6994. doi:10.1128/Jb.00707-06
Librando V, Forte S (2005) Computer evaluation of protein segments removal effects from naphthalene 1,2-dioxygenase enzyme on polycyclic aromatic hydrocarbons interaction. Biochem Eng J 27(2):161–166. doi:10.1016/j.bej.2005.08.011
Parales R, Gibson D, Resnick S, Lee K (2000) Novel naphthalene dioxygenase and methods for their use. WO Patent WO 2000/037480 A1
Librando V, Pappalardo M (2012) Engineered enzyme interactions with polycyclic aromatic hydrocarbons: a theoretical approach. J Mol Graph Model 36:30–35. doi:10.1016/j.jmgm.2012.02.005
Librando V, Pappalardo M (2011) Computational study on the interaction of a ring-hydroxylating dioxygenase from Sphingomonas CHY-1 with PAHs. J Mol Graph Model 29(7):915–919. doi:10.1016/j.jmgm.2011.03.001
Karlsson A, Parales JV, Parales RE, Gibson DT, Eklund H, Ramaswamy S (2003) Crystal structure of naphthalene dioxygenase: side-on binding of dioxygen to iron. Science 299(5609):1039–1042. doi:10.1126/science.1078020
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38, 27–38
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26(16):1781–1802. doi:10.1002/jcc.20289
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Jinno K (2001) Polycyclic Aromatic Hydrocarbons (PAHs) database. http://chrom.tutms.tut.ac.jp/JINNO/DATABASE/00alphabet.html#HEAD
Gasteiger J, Marsili M, Hutchings MG, Saller H, Low P, Rose P, Rafeiner K (1990) Models for the representation of knowledge about chemical-reactions. J Chem Inf Comput Sci 30(4):467–476. doi:10.1021/Ci00068a019
Trott O, Olson AJ (2010) Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. doi:10.1002/Jcc.21334
Toth D, Franco J (2012) Using supercomputing to conduct virtual screen as part of the drug discovery process in a medicinal chemistry course. J Comput Sci Educ 3(2):8
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98(18):10037–10041. doi:10.1073/pnas.181342398
Medek P, Benes P, Sochor J (2007) Computation of tunnels in protein molecules using Delaunay triangulation. J Wscg 15(1–3):107–114
White SH, Wimley WC (1999) Membrane protein folding and stability: physical principles. Annu Rev Bioph Biom 28:319–365. doi:10.1146/annurev.biophys.28.1.319
Wimley WC, White SH (1999) Calorimetric studies of beta-sheet folding in membranes. Biophys J 76(1):A176–A176
Acknowledgments
This work was funded by the Italian Ministry of Education, University and Research (MIUR), PRIN 2009, and the COMETA Consortium.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1627 kb)
Rights and permissions
About this article
Cite this article
Librando, V., Pappalardo, M. Theoretical approach to the innovative mutation of naphthalene 1,2-dioxygenase: a molecular dynamics and docking study. J Mol Model 20, 2354 (2014). https://doi.org/10.1007/s00894-014-2354-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2354-x