Abstract
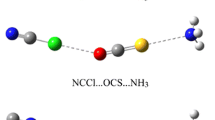
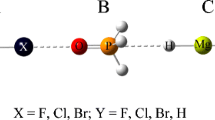
The M∙∙∙HCCX∙∙∙NH3 (M = Li+, Na+, Cu+, Ag+, Au+; X = Cl, Br) complexes were designed to study the influence of cation–π interaction on the X∙∙∙N halogen bonds under M05-2X/aug-cc-pVDZ(PP) level. In comparison with the HCCX∙∙∙NH3 complexes, the bond distances of the halogen bonds have decreased, and the interaction energies become more negative. The results show that the X∙∙∙N halogen bonds have been strengthened by the cation−π interactions. For different cations, the enhancing effect is more intensive in the order of Au+ > Cu+ > Ag+ > Li+ > Na+, which indicates that transition metal cations can enhance the halogen bond in a stronger manner. Molecular electrostatic potential and second-order perturbation stabilization energy were calculated to deepen the discussion. In addition, atoms in molecules analysis was performed and the electron density shift was studied.




Similar content being viewed by others
Reference
Chalasinski G, Szczesniak MM (2000) Chem Rev 100:4227–4252
Rudkevich DM (2004) Angew Chem Int Ed 43:558–571
Saalfrank RW, Maid H, Scheurer A (2008) Angew Chem Int Ed 47:8794–8824
Metrangolo P, Resnati G (eds) (2007) Halogen bonding: fundamentals and applications, structure and bonding. Springer, Berlin
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Politzer P, Lane P, Concha MC, Ma YG, Murray JS (2007) J Mol Model 13:305–311
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748–7757
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Comput Theor Chem 998:2–8
Politzer P, Murray JS (2013) Chem Phys Chem 17:278–294
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178–11189
Politzer P, Murray JS (2013) Cryst Eng Comm 15:3145–3150
Metrangolo P, Resnati G (2001) Chem Eur J 7:511–2519
Corradi E, Meille SV, Messina MT, Metrangolo P, Resnati G (2000) Angew Chem Int Ed 39:1782–1786
Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G (2008) Angew Chem Int Ed 47:6114–6127
Loc Nguyen H, Horton PN, Hursthouse MB, Legon AC, Bruce DW (2004) J Am Chem Soc 126:16–17
Legon AC (2010) Phys Chem Chem Phys 12:7736–7747
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:386–395
Cavallo G, Metrangolo P, Pilati T, Resnati G, Sansotera M, Terraneo G (2010) Chem Soc Rev 39:3772–3783
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Proc Natl Acad Sci U S A 101:16789–16794
Parisini E, Metrangolo P, Pilati T, Resnati G, Terraneo G (2011) Chem Soc Rev 40:2267–2278
Lu YX, Shi T, Wang Y, Yang HY, Yan XH, Luo XM, Jiang HL, Zhu WL (2009) J Med Chem 52:2854–2862
Lu YX, Wang Y, Zhu WL (2010) Phys Chem Chem Phys 12:4543–4551
Vijay D, Sastry GN (2010) Chem Phys Lett 485:235–242
Parra RD, Ohlssen J (2008) J Phys Chem A 112:3492–3498
Egi M, Sarkhel S (2007) Acc Chem Res 40:197–205
Alkorta I, Blanco F, Elguero J (2008) J Phys Chem A 112:6753–6759
Politzer P, Murray JS, Concha MC (2007) J Mol Model 13:643–650
Alkorta I, Blanco F, Elguero J, Estarellas C, Frontera A, Quinonero D, Deya PM (2009) J Chem Theor Comput 5:1186–1194
Frontera A, Quinonero D, Costa A, Ballester P, Deya PM (2007) New J Chem 31:556–560
Estarellas C, Frontera A, Quinonero D, Alkorta I, Deya PM, Elguero J (2009) J Phys Chem A 113:3266–3273
Lankau T, Wu YC, Zou JW, Yu CH (2008) J Theor Comp Chem 7:13–35
Politzer P, Murray JS, Lane P (2007) Int J Quantum Chem 107:3046–3052
Zhao Q, Feng DC, Hao JC (2011) J Mol Model 17:2817–2823
Lu YX, Liu YT, Li HY, Zhu X, Liu HL, Zhu WL (2012) J Phys Chem A 116:2591–2597
Li HY, Lu YX, Liu YT, Zhu X, Liu HL, Zhu WL (2012) Phys Chem Chem Phys 14:9948–9955
Alkorta I, Rozas I, Elguero J (1998) Theor Chem Acc 99:116–123
Mahadevi AS, Sastry GN (2013) Chem Rev 113:2100–2138
Li R, Li QZ, Chen JB, Liu ZB, Li WZ (2011) Chem Phys Chem 11:2289–2295
Lu YX, Liu YT, Li HY, Zhu X, Liu HL, Zhu WL (2012) Chem Phys Chem 13:2154–2161
Zhao Y, Truhlar DG (2006) J Chem Theory Comput 2:1009–1018
Zhao Y, Truhlar DG (2008) Acc Chem Res 41:157–167
Wang WZ, Zhang Y, Ji BM, Tian AM (2011) J Chem Phys 134:224303–224307
Zhang Y, Ma N, Wang WZ (2012) Chem Phys Lett 532:27–30
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Frisch MJ et al. (2009) Gaussian 09 (Revision B.01). Gaussian Inc, Pittsburgh
Reed AE, Curtiss LA, Weinhold F (1998) Chem Rev 88:899–926
Lu T, Chen FW (2012) J Comp Chem 33:580–592
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, New York
Todd A Keith (2013) AIM All version 13.05.06, aim.tkgristmill.com
Li QZ, Dong X, Jing B, Li WZ, Chen JB, Gong BA, Yu ZW (2010) J Comput Chem 31:1662–1669
Acknowledgments
The author is grateful to help of high performance computing centre in Shandong University and reasonable advice of Prof. Feng in Shandong University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geng, D. Theoretical investigations on the enhancing effect of the cation–π interaction on the halogen bond in the M∙∙∙HCCX∙∙∙NH3 (M = Li+, Na+, Cu+, Ag+, Au+; X = Cl, Br) complexes. J Mol Model 20, 2235 (2014). https://doi.org/10.1007/s00894-014-2235-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2235-3