Abstract
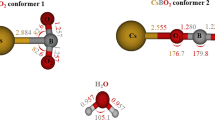
Structure and thermodynamic properties (standard enthalpies of formation and Gibbs free energies) of hydrated caesium species of nuclear safety interest, Cs, CsOH, CsI and its dimer Cs2I2, with one up to three water molecules, are calculated to assess their possible existence in severe accident occurring to a pressurized water reactor. The calculations were performed using the coupled cluster theory including single, double and non-iterative triple substitutions (CCSD(T)) in conjunction with the basis sets (ANO-RCC) developed for scalar relativistic calculations. The second-order spin-free Douglas-Kroll-Hess Hamiltonian was used to account for the scalar relativistic effects. Thermodynamic properties obtained by these correlated ab initio calculations (entropies and thermal capacities at constant pressure as a function of temperature) are used in nuclear accident simulations using ASTEC/SOPHAEROS software. Interaction energies, standard enthalpies and Gibbs free energies of successive water molecules addition determine the ordering of the complexes. CsOH forms the most hydrated stable complexes followed by CsI, Cs2I2, and Cs. CsOH still exists in steam atmosphere even at quite high temperature, up to around 1100 K.







Similar content being viewed by others
References
Insights into the Control of the Release of Iodine, Cesium, Strontium and other Fission Products in the Containment by Severe Accident Management, NEA/CSNI/R(2000)9. Nuclear Energy Agency. http://www.oecd-nea.org/nsd/docs/2000/csni-r2000-9.pdf. Accessed 24 January 2012
Clément B, Cantrel L, Ducros G, Funke F, Herranz L, Rydl A, Weber G, Wren C (2007) State of the Art Report on Iodine Chemistry, NEA/CSNI/R(2007)1. Nuclear Energy Agency. http://www.oecd-nea.org/nsd/docs/2007/csni-r2007-1.pdf. Accessed 24 January 2012
Kissane MP (2009) A review of radionuclide behaviour in the primary system of a very-high-temperature reactor. Nucl Eng Des 239(12):3076–3091
Pontillon Y, Ducros G (2010) Behaviour of fission products under severe PWR accident conditions: The VERCORS experimental programme. Part 2: Release and transport of fission gases and volatile fission products. Nucl Eng Des 240(7):1853–1866
Canneaux S, Sokolowski-Gomez N, Henon E, Bohr F, Dóbé S (2004) Theoretical study of the reaction OH + acetone: a possible kinetic effect of the presence of water. PCCP 6(22):5172–5177
Iuga C, Alvarez-Idaboy JR, Reyes L, Vivier-Bunge A (2010) Can a Single Water Molecule Really Catalyze the Acetaldehyde + OH Reaction in Tropospheric Conditions? J Phys Chem Lett 1(20):3112–3115
Mansergas A, González J, Ruiz-López M, Anglada JM (2011) The gas phase reaction of carbonyl oxide with hydroxyl radical in presence of water vapor. A theoretical study on the reaction mechanism. Comput Theor Chem 965(2–3):313–320
Kuczkowski RL, Lide DR Jr (1966) Microwave Spectra of Alkali Hydroxides: Evidence for Linearity of CsOH and KOH. J Chem Phys 44:3131–3132
Lide DR Jr, Kuczkowski RL (1967) Structure of the Alkali Hydroxides. I. Microwave Spectrum of Gaseous CsOH. J Chem Phys 46:4768–4774
Acquista N, Abramowitz S, Lide DR (1968) Structure of the Alkali Hydroxides. II. The Infrared Spectra of Matrix-Isolated CsOH and CsOD. J Chem Phys 49(2):780–782
Lide DR Jr, Matsumura C (1969) Structure of the Alkali Hydroxides. IV. Interpretation of Vibration-Rotation Interactions in CsOH and RbOH and Refinement of Structures. J Chem Phys 50(7):3080–3086
Story TL, Hebert AJ (1976) Dipole moments of KI, RbBr, RbI, CsBr, and CsI by the electric deflection method. J Chem Phys 64(2):855–858
Tzu-Min Su R, Riley SJ (1979) Alkali halide photofragment spectra. I. Alkali iodide bond energies and excited state symmetries at 266 nm. J Chem Phys 71(8):3194–3202
Blackburn PE, Johnson CE (1988) Mass Spectrometry Studies of Fission Product Behavior II. Gas phase species in the CsI-CsOH system. J Nucl Mater 154:74–82
Li R-Z, Liu C-W, Gao YQ, Jiang H, Xu H-G, Zheng W-J (2013) Microsolvation of LiI and CsI in Water: Anion Photoelectron Spectroscopy and ab initio Calculations. J Am Chem Soc 135(13):5190–5199
Inada Y, Akagane K (1996) Non-Empirical Study of Chemical Reactions Including Fission Products in Severe Light Water Reactor Accidents. J Nucl Sci Technol 33(7):562–568
Inada Y (1998) Non-Empirical Analysis of the Chemical Reaction of Cesium with Steam; Cs + H2O - > CsOH + H, in Sever Light Water Reactor Accidents. J Nucl Sci Technol 35(4):313–316
Lee EPF, Wright TG (2003) Theoretical Study of RbOH, CsOH, FrOH, and Their Cations: Geometries, Vibrational Frequencies, and the Ionization Energies. J Phys Chem A 107(26):5233–5240
Kurosaki Y, Matsuoka L, Yokoyama K, Yokoyama A (2008) Ab initio study on the ground and low-lying excited states of cesium iodide (CsI). J Chem Phys 128(2):024301
Badawi M, Xerri B, Canneaux S, Cantrel L, Louis F (2012) Molecular structures and thermodynamic properties of 12 gaseous cesium-containing species of nuclear safety interest: Cs2, CsH, CsO, Cs2O, CsX, and Cs2X2 (X = OH, Cl, Br, and I). J Nucl Mater 420(1–3):452–462
Odde S, Pak C, Lee HM, Kim KS, Mhin BJ (2004) Aqua dissociation nature of cesium hydroxide. J Chem Phys 121(1):204–208
Kolaski M, Lee HM, Choi YC, Kim KS, Tarakeshwar P, Miller DJ, Lisy JM (2007) Structures, energetics, and spectra of aqua-cesium (I) complexes: An ab initio and experimental study. J Chem Phys 126(7):074302
Ali SM, De S, Maity DK (2007) Microhydration of Cs+ ion: A density functional theory study on Cs+-(H2O)n clusters (n = 1-10). J Chem Phys 127(4):044303
Streitwieser A, Liang JC-Y, Jayasree EG, Hasanayn F (2007) Evaluation of Two Computational Models Based on Different Effective Core Potentials for Use in Organocesium Chemistry. J Chem Theory Comput 3(1):127–131
Cousin F, Dieschbourg K, Jacq F (2008) New capabilities of simulating fission product transport in circuits with ASTEC/SOPHAEROS v. 1.3. Nucl Eng Des 238:2430–2438
Van Dorsselaere JP, Seropian C, Chatelard P, Jacq F, Fleurot J, Giordano P, Reinke N, Schwinges B, Allelein HJ, Luther W (2009) The ASTEC Integral Code for Severe Accident Simulation. Nucl Technol 165:293–307
Møller C, Plesset MS (1934) Note on an Approximation Treatment for Many-Electron Systems. Phys Rev 46(7):618–622
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford, CT
Kendall RA, Dunning JTH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96(9):6796–6806
Peterson KA, Shepler BC, Figgen D, Stoll H (2006) On the spectroscopic and thermochemical properties of ClO, BrO, IO, and their anions. J Phys Chem A 110(51):13877–13883
Lim IS, Schwerdtfeger P, Metz B, Stoll H (2005) All-electron and relativistic pseudopotential studies for the group 1 element polarizabilities from K to element 119. J Chem Phys 122(10):104103
Sudolská M, Cantrel L, Budzák Š, Černušák I (2014) Molecular structures and thermodynamic properties of monohydrated gaseous iodine compounds: Modelling for severe accident simulation. J Nucl Mater 446(1–3):73–80
Perdew JP, Burke K, Ernzerhof M (1996) Generalized Gradient Approximation Made Simple. Phys Rev Lett 77(18):3865–3868
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
VandeVondele J, Hutter J (2007) Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J Chem Phys 127(11):114105
Goedecker S, Teter M, Hutter J (1996) Separable dual-space Gaussian pseudopotentials. Phys Rev B 54(3):1703–1710
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126(1):014101
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput Phys Commun 167(2):103–128
Louis F, Černušák I, Canneaux S, Mečiarová K (2011) Atmospheric reactivity of CH3I and CH2I2 with OH radicals: A comparative study of the H- versus I-abstraction. Comput Theor Chem 965(2–3):275–284
Watts JD, Gauss J, Bartlett RJ (1983) Coupled-cluster methods with noniterative triple excitations for restricted open-shell Hartree-Fock and other general single determinant reference functions. Energies and analytical gradients. J Chem Phys 98(11):8718–8733
.Aquilante F, De Vico L, Ferré N, Ghigo G, Malmqvist P-Å, Neogrády P, Pedersen TB, Pitoňák M, Reiher M, Roos BO, Serrano-Andrés L, Urban M, Veryazov V, Lindh R (2010) MOLCAS 7: The Next Generation. J Comput Chem 31(1):224–247
Veryazov V, Widmark P-O, Serrano-Andres L, Lindh R, Roos BO (2004) 2MOLCAS as a development platform for quantum chemistry software. Int J Quantum Chem 100(4):626–635
Karlström G, Lindh R, Malmqvist P-Å, Roos BO, Ryde U, Veryazov V, Widmark P-O, Cossi M, Schimmelpfennig B, Neogrady P, Seijo L (2003) MOLCAS: a program package for computational chemistry. Comput Mater Sci 28(2):222–239
Roos BO, Lindh R, Malmqvist P-Å, Veryazov V, Widmark P-O (2005) Main Group Atoms and Dimers Studied with a New Relativistic ANO Basis Set. J Phys Chem A 108(15):2851–2858
Widmark P-O, Malmqvist P-Å, Roos BO (1990) Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions I. First row atoms. Theor Chem Acc 77(5):291–306
Dunning JTH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566
Xantheas SS (1996) On the importance of the fragment relaxation energy terms in the estimation of the basis set superposition error correction to the intermolecular interaction energy. J Chem Phys 104(21):8821–8824
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys 110(13):6158–6170
Becke AD (1997) Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J Chem Phys 107(20):8554–8560
Schmider HL, Becke AD (1998) Optimized density functionals from the extended G2 test set. J Chem Phys 108(23):9624–9631
Krishnan R, Pople JA (1978) Approximate fourth-order perturbation theory of the electron correlation energy. Int J Quantum Chem 14(1):91–100
Krishnan R, Frisch MJ, Pople JA (1980) Contribution of triple substitutions to the electron correlation energy in fourth order perturbation theory. J Chem Phys 72(7):4244–4245
Klimeš J, Michaelides A (2012) Perspective: Advances and challenges in treating van der Waals dispersion forces in density functional theory. J Chem Phys 137(12):120901
Acknowledgments
We appreciate the support from the Slovak Research and Development Agency under the contract No. APVV-0059-10 and from the Comenius University Grant GUK/38/2013. Part of this work was done under the Research & Development Operational Program funded by the ERDF: Amplification of the Centre of Excellence on Green Chemistry Methods and Processes (CEGreen-II 26240120025). MS thanks SARNET for the support of her stay in Cadarache. A part of this work was carried out using the HELIOS supercomputer system at Computational Simulation Centre of International Fusion Energy Research Centre (IFERC-CSC), Aomori, Japan, under the Broader Approach collaboration between Euratom and Japan. This work has also beneficiated of the HPC resources from GENCI-IDRIS (Grant 2013-project number x2013086731).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection 9th European Conference on Computational Chemistry (EuCo-CC9).
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 564 kb)
Rights and permissions
About this article
Cite this article
Sudolská, M., Cantrel, L. & Černušák, I. Microhydration of caesium compounds: Cs, CsOH, CsI and Cs2I2 complexes with one to three H2O molecules of nuclear safety interest. J Mol Model 20, 2218 (2014). https://doi.org/10.1007/s00894-014-2218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2218-4