Abstract
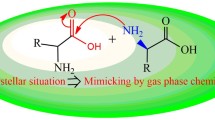
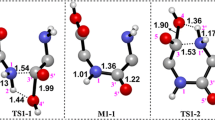
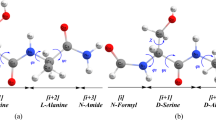
In this work, peptide bond cleavages at carboxy- and amino-sides of the aspartic residue in a peptide model via direct (concerted and step-wise) and cyclic intermediate hydrolysis reaction pathways were explored computationally. The energetics, thermodynamic properties, rate constants, and equilibrium constants of all hydrolysis reactions, as well as their energy profiles were computed at the B3LYP/6-311++G(d,p) level of theory. The result indicated that peptide bond cleavage of the Asp residue occurred most preferentially via the cyclic intermediate hydrolysis pathway. In all reaction pathways, cleavage of the peptide bond at the amino-side occurred less preferentially than at the carboxy-side. The overall reaction rate constants of peptide bond cleavage of the Asp residue at the carboxy-side for the assisted system were, in increasing order: concerted < step-wise < cyclic intermediate.











Similar content being viewed by others
References
Pan B, Ricci MS, Trout BL (2006) Biochemistry 45:15430–15443
Chu JW, Yin J, Brooks BR, Wang DIC, Ricci MS, Brems DN, Trout BLJ (2004) Pharm Sci 93:3096–3102
Liu DTY (1992) Trends Biotechnol 10:364–369
Kosky AA, Razzaq UO, Treuheit MJ, Brems DN (1999) Protein Sci 8:2519–2523
Wei W (1999) Int J Pharm 185:129–188
Krug JP, Popelier PLA, Bader RFW (1992) J Phys Chem 96:7604–7616
Antonczak S, Ruizlopez MF, Rivail JL (1994) J Am Chem Soc 116:3912–3921
Bakowies D, Kollman PA (1999) J Am Chem Soc 121:5712–5726
Kahne D, Still WC (1988) J Am Chem Soc 110:7529–7534
Brown RS, Bennet AJ, Slebockatilk H (1992) Acc Chem Res 25:481–488
Bryant RAR, Hansen DE (1996) J Am Chem Soc 118:498–5499
Radzicka A, Wolfenden R (1996) J Am Chem Soc 118:6105–6109
Gorb L, Asensio A, Tunon I, Ruiz-Lopez MF (2005) Chem Eur J 11:6743–6753
Cascella M, Raugei S, Carloni P (2004) J Phys Chem B 108:369–375
Zahn D (2004) Eur J Org Chem 19:4020–4023
Pan B, Ricci MS, Trout BL (2011) J Phys Chem B 115:5958–5970
Pan B, Ricci MS, Trout BL (2010) J Phys Chem B 114:4389–4399
Wang B, Cao Z (2010) J Phys Chem A 114:12918–12927
Catak S, Monard G, Aviyente V, Ruiz-Lopez MR (2008) J Phys Chem A 112:8752–8761
Catak S, Monard G, Aviyente V, Ruiz-Lopez MR (2006) J Phys Chem A 110:8354–8365
Joshi AB, Kirch LE (2004) Int J Pharm 273:213–219
Joshi AB, Rus E, Kirch LE (2000) Int J Pharm 203:115–125
Joshi AB, Sawai M, Kearny WR, Kirch LE (2005) J Pharm Sci 94:1912–1927
Herrman KA, Wysocski VH (2005) J Am Soc Mass Spectrom 16:1067–1080
Oliyai C, Borchardt RT (1993) Pharm Res 10:95–102
Stewart JJP (1989) J Comput Chem 10:221–264
Parr RG, Young W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford
Hohenberg P, Kohn W (1964) Phys Rev B 136:864–871
Khon W, Sham L (1965) J Phys Rev A 140:1133–1138
Beck AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr R (1988) Phys Rev B 37:785–789
Madura J, Jorgensen WL (1986) J Am Chem Soc 108:2517–2527
Tomasi J, Mennucci B, Cancés E (1999) J Mol Struct (THEOCHEM) 464:211–226
Cancès ET, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Mennucci B, Tomasi J (1997) J Chem Phys 106:5151–5158
Mennucci B, Cancès ET, Tomasi J (1997) J Phys Chem B 101:10506–10517
Cossi M, Barone V (1998) J Chem Phys 109:6246–6254
Barone V, Cossi M, Tomasi J (1997) J Chem Phys 107:3210–3221
Cossi M, Scalmani G, Rega N, Barone V (2002) J Chem Phys 117:43–54
Frisch MJ et al (2003) Gaussian 03. Revision B.03. Gaussian, Pittsburgh
Flükiger P, Lüthi HP, Portmann S, Weber J (2000) MOLEKEL 4.3. Swiss Center for Scientific Computing, Manno
Ochterski JW (2000) Thermochemistry in Gaussian. Gaussian, Pittsburgh
Ruangpornvisuti V (2009) Int J Quant Chem 109:275–284
Bravo-Perez G, Alvarez-Idaboy JR, Cruz-Torres A, Ruiz ME (2002) Phys Chem A 106:4645–4650
Wigner EZ (1932) Phys Chem B 19:203–216
Hirschfelder JO, Wigner E (1939) J Chem Phys 7:616–628
Bell RP (1980) The tunnel effect in chemistry. Chapman and Hall, London
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Sang-aroon W, Ruangpornvisuti V (2013) J Mol Model 19(9):3627–3636
DaCosta H, Fan M (2012) Rate constant calculation for thermal reactions: methods and applications. Wiley, Hoboken, NJ
Zhang S, Basile FJ (2007) Proteome Res 6:1700–1704
Loudon GM (1983) Organic chemistry. Addison-Wesley, Reading, MA
Geiger T, Clarke S (1987) J Biol Chem 262:785–794
Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW (1988) J Biol Chem 263:19020–19023
Acknowledgments
This research work was supported financially by The Thailand Research Fund, co-funded by The Commission of Higher Education and The Faculty of Engineering, Rajamangala University of Technology Isan, Khonkaen campus through the young academic research grant no. MRG5380243 to W.S., which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sang-aroon, W., Amornkitbamrung, V. & Ruangpornvisuti, V. A density functional theory study on peptide bond cleavage at aspartic residues: direct vs cyclic intermediate hydrolysis. J Mol Model 19, 5501–5513 (2013). https://doi.org/10.1007/s00894-013-2054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-2054-y