Abstract
The reaction mechanisms involved in the scavenging of hydroxyl (OH·), methoxy (OCH3 ·), and nitrogen dioxide (NO2 ·) radicals by ellagic acid and its monomethyl and dimethyl derivatives were investigated using the transition state theory and density functional theory. The calculated Gibbs barrier energies associated with the abstraction of hydrogen from the hydroxyl groups of ellagic acid and its monomethyl and dimethyl derivatives by an OH· radical in aqueous media were all found to be negative. When NO2 · was the radical involved in hydrogen abstraction, the Gibbs barrier energies were much larger than those calculated when the OH· radical was involved. When OCH3 · was the hydrogen-abstracting radical, the Gibbs barrier energies lay between those obtained with OH· and NO2 · radicals. Therefore, the scavenging efficiencies of ellagic acid and its monomethyl and dimethyl derivatives towards the three radicals decrease in the order OH· >> OCH3 · > NO2 ·. Our calculated rate constants are broadly in agreement with those obtained experimentally for hydrogen abstraction reactions of ellagic acid with OH· and NO2· radicals.

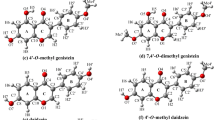
Reactant complex (RC), transition state (TS), and product complex (PC) for hydrogen abstraction from ellagic acid by an OH· radical




Similar content being viewed by others
References
Wiseman H, Halliwell B (1996) J Biochem 313:17–29
Marnett LJ (2000) Carcinogenesis 21:361–370
Hussain SP, Hofseth LJ, Harris CC (2003) Nat Rev Cancer 3:276–285
Loft S, Poulsen HE (2006) J Mol Med 74:297–312
Doll R, Peto R (1981) J Natl Cancer Inst 66:1191–1308
Chakraborty P, Kumar S, Dutta D, Gupta V (2009) J Pharm Tech 2:238–244
Ames BN, Shigenaga MK, Hagen TM (1993) Proc Natl Acad Sci U S A 90:7915–7922
Mecocci P, Fano G, Fulle S, Macgarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF (1999) Free Rad Biol Med 26:303–308
Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF (1993) Annal Neurol 34:609–616
Squadrito GL, Pryor WA (1998) Free Rad Biol Med 25:392–403
Halliwell B, Gutterridge JMC (1984) J Biochem 219:1–14
Yarkony DR, Schaefer HF, Rothenberg S (1974) J Am Chem Soc 96:656–659
Halliwell B (1999) Mut Res 443:37–52
Pryor WA, Stone K (1993) Ann NY Acad Sci 686:12–28
Augusto O, Bonini MG, Amanso AM, Linares E, Santos CCX, Menezes LD (2002) Free Rad Biol Med 32:841–859
Shukla PK, Mishra PC (2008) J Phys Chem B 112:4779–4789
Niles JC, Wishnok JS, Tannenbaum SR (2006) Nitric Oxide 14:109–121
Sodum RS, Fiala ES (2001) Chem Res Toxicol 14:438–450
Pavlovic R, Santaniello E (2007) J Pharm Pharmacol 59:1687–1695
Jena NR, Mishra PC, Suhai S (2009) J Phys Chem B 113:5633–5644
Agnihotri N, Mishra PC (2009) J Phys Chem B 113:3129–3138
Agnihotri N, Mishra PC (2010) J Phys Chem B 114:7391–7404
Shukla MK, Mishra PC (1995) Spectrochim Acta A 51:831–838
Agnihotri N, Mishra PC (2009) J Phys Chem B 113:12096–12104
Tiwari S, Mishra PC (2011) J Mol Model 17:59–72
Shukla MK, Mishra PC (1996) J Mol Struct (Theochem) 377:247–259
Shukla PK, Mishra PC (2007) J Phys Chem B 111:4603–4615
Yadav A, Mishra PC (2012) Chem Phys 405:76–88
Yadav A, Mishra PC (2013) J Mol Model 19:767–777
Galano A, Alvarez-Idaboy JR (2011) R Soc Chem 1:1763–1771
Agnihotri N, Mishra PC (2011) J Phys Chem A 115:14221–14232
Mates JM, Perez-Gomez C, de Castro IN (1999) Clin Biochem 32:595–603
Mullen W, McGinn J, Lean ME, MacLean MR, Gardner P, Duthie GG, Yokota T, Crozier A (2002) J Agric Food Chem 50:5191–5196
Radtke J, Linseisen J, Wolfram G (1998) Ernaehrungswiss 37:190–197
Mohajeri A, Asemani SS (2009) J Mol Struct 930:15–20
Vattem DA, Shetty K (2005) J Food Biochem 29:234–266
Da Silva SL, Calgarotto AK, Chaar JS, Marangoni S (2008) Toxicon 52:655–666
Zafrilla P, Ferreres F, Francisco AT (2001) J Agric Food Chem 49:3651–3655
Ancos B, Gonzalez EM, Cano P (2000) J Agric Food Chem 48:4565–4570
Hakkinen SH, Karenlampi SO, Mykkanen H, Heinonen IM, Torronen AR (2000) Eur Food Res Tech 212:75–80
Daniel EM, Krupnick AS, Heur YH, Blinzler JA, Nims RW, Stoner GD (1989) J Food Comp Anal 2:385–398
Goldberg DM, Hoffman B, Yang J, Soleas GJ (1999) J Agric Food Chem 47:3978–3985
Bobinaite R, Viskelis P, Venskutonis PR (2012) Food Chem 132:1495–1501
Zhang J, Xiong Y, Peng B, Gao H, Zhou Z (2011) Comp Theo Chem 963:148–153
Teel RW, Babcock MS, Dixit R, Stoner GD (1986) Cell Biol Toxicol 2:53–62
Zhang Z, Hamilton SM, Stewart C, Strother A, Teel RW (1993) Anticancer Res 13:2341–2346
Wood AW, Huang MT, Chang RL, Newmark HL, Lehr RE, Yagi H, Sayer JM, Jerina DM, Conney AH (1982) Proc Natl Acad Sci USA 79:5513–5517
Hassoun EA, Walter AC, Alsharif NZ, Stohs SJ (1997) Toxicology 124:27–37
Priyadarsini KI, Khopde SM, Santosh SK, Mohan H (2002) J Agric Food Chem 50:2200–2206
Barch DH, Rundhaugen LM, Stoner GD, Pillay NS, Rosche WA (1996) Carcinogenesis 17:265–269
Muthenna P, Akileshwari C, Reddy GB (2012) J Biochem 442:221–230
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Miertus S, Tomasi J (1982) Chem Phys 65:239–245
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358–1371
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796–6806
Perdew JP, Burke K, Wang Y (1996) Phys Rev B 54:16533–16539
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson, Patparganj
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem, Shawnee Mission
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A1. Gaussian, Wallingford
Silva PJ, Ramos MJ (2011) Comp Theo Chem 966:120–126
Ess DH, Houk KN (2005) J Phys Chem A 109:9542–9553
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem A 111:10439–10452
Rossi M, Erlebacher J, Zacharias DE, Carrel HL, Iannucci B (1991) Carcinogenesis 12:2227–2232
Wright JS, Johnson ER, DiLabio GA (2001) J Am Chem Soc 123:1173–1183
Acknowledgments
The authors are thankful to the University Grants Commission (New Delhi) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.86 MB)
Rights and permissions
About this article
Cite this article
Tiwari, M.K., Mishra, P.C. Modeling the scavenging activity of ellagic acid and its methyl derivatives towards hydroxyl, methoxy, and nitrogen dioxide radicals. J Mol Model 19, 5445–5456 (2013). https://doi.org/10.1007/s00894-013-2023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-2023-5