Abstract
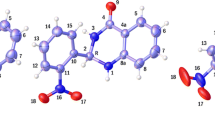
In the present work, we carried out a conformational analysis of cis-3-aminoindan-1-ol and evaluated the role of the intramolecular hydrogen bond in the stabilization of various conformers using quantum mechanical DFT (B3LYP) and MP2 methods. On the basis of relative energies, we have found nine conformational minima, which can interchange through the ring-puckering and the internal rotation of the OH and NH2 groups on the five-membered ring. The intramolecular hydrogen bonds such as OH∙∙∙∙π, NH∙∙∙∙π, NH∙∙∙∙OH and HN∙∙∙∙HO are expected to be of critical importance for the conformational stabilities. The intramolecular interactions of the minima have been analyzed by calculation of electron density (ρ) and Laplacian (ρ) at the bond critical points (BCPs) using atoms-in-molecule (AIM) theory. The existence or absence of OH∙∙∙∙π and NH∙∙∙∙π in cis-3-aminoindan-1-ol remains unclear since the geometrical investigation has not been confirmed by topological criteria. The results of theoretical calculations demonstrate that this compound exists predominantly in one ring-puckering form stabilized by strong hydrogen bond HN∙∙∙∙HO Interaction.





Similar content being viewed by others
References
Lûpez-Garcìa M, Alfonso I, Gotor V (2004) Chem Eur J 10:3006–3014
Vieth M, Cummins DJ (2000) J Med Chem 43:3020–3032
Pÿrez C, Pastor M, Ortiz AR, Gago F (1998) J Med Chem 41:836–852
Froimowitz M, Wu KM, Moussa A, Haidar RM, Juravyj J, George C, Gardner EL (2000) J Med Chem 43:4981–4992
Gilad GM, Gilad VH (1999) J Pharmacol Exp Therap 291:39–43
Szulc ZM, Hannun YA, Bielawska A (2000) Tetrahedron Lett 41:7821–7824
Kawabata T, Yamamoto K, Momose Y, Yoshida H, Nagaoka Y, Fuji K (2001) Chem Commun 2700–2701
Lait SM, Rankic DA, Keay BA (2007) Chem Rev 107:767–796
Balbás IM, Mendoza BED, Fernández-Zertuche M, Ordoñez M, Linzaga-Elizalde I (2012) Molecules 17:151–162
André C, Calmès M, Escale F, Amblard M, Martinez J, Songis O (2012) Amino Acids 43:415–421
Kinbara K, Katsumata Y, Saigo K (2002) Chem Lett 3:266–267
Ottaviani P, Velino B, Caminati W (2006) J Mol Struct 795:194–197
Al-Saadi AA, Wagner M, Laane J (2006) J Phys Chem A 110:12292–12297
Al-Saadi AA, Ocola EJ, Laane J (2010) J Phys Chem A 114:7453–7456
Hamza A (2010) Struct Chem 21:939–945
Iga H, Isozaki T, Suzuki T, Ichimura T (2007) J Phys Chem A 111:5981–5987
Velino B, Ottaviani P, Caminati W, Giardini A, Paladini A (2006) Chem Phys Chem 7:565–568
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press Inc., New York
Guemmour H, Kheffache D, Benaboura A (2011) J Mol Strut 1002:151–158
Le Barbu-Debus K, Lahmani F, Zehnacker-Rentien A, Guchhait K (2006) Chem Phys Lett 422:218–225
Bader RFW, Streitwieser A, Neuhaus A, Laidig KE, Speers P (1996) J Am Chem Soc 118:4959–4965
Carrol MT, Chang C, Bader RFW (1988) Mol Phys 63:387–405
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03 Revision B.04. Gaussian Inc, Wallingford
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Helgaker T, Jorgensen P, Olsen J (2000) Molecular Electronic-Structure Theory. Wiley, New York
Lu T, Chen F (2012) J Comput Chem 33:580–592
Koch U, Popelier P (1995) J Phys Chem 99:9747–9754
Popelier PLA (1998) J Phys Chem A 102:1873
McMahan MA, Sharma SD, Curl RF Jr (1979) J Mol Spectrosc 75:220–233
van Mourik T, Gdanitz RJ (2002) J Chem Phys 116:9620–9623
Miller BJ, Lane JR, Kjaergaard HG (2011) Phys Chem Chem Phys 13:14183–14193
Lane JR, Contreras-García J, Piquemal J-P, Miller BJ, Kjaergaard HG (2013) J Chem Theory Comput 9:3263–3266
Contreras-Garcia J, Yang W, Johnson ER (2011) J Phys Chem A 115:12983–12990
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Politzer P, Murray JS (2002) Theor Chem Acc 108:134–142
Varetto U MOLEKEL 5.4.0.8; Swiss National Supercomputing Centre: Lugano, Switzerland
Alonso JL, Pérez C, Sanz ME, López JC, Blanco S (2009) Phys Chem Chem Phys 11:617–627
Isozaki T, Iga H, Suzuki T, Ichimura T (2007) J Chem Phys 126:214–304
Laane J, Bondoc E, Sakurai S, Morris K, Meinander N, Choo J (2000) J Am Chem Soc 122:2628–2634
Acknowledgments
The reviewers of this manuscript are acknowledged for their helpful suggestions
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kheffache, D., Guemmour, H., Dekhira, A. et al. Conformational analysis and intramolecular hydrogen bonding of cis-3-aminoindan-1-ol: a quantum chemical study. J Mol Model 19, 4837–4847 (2013). https://doi.org/10.1007/s00894-013-1989-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1989-3