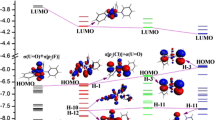
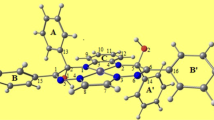
Abstract
The potential energy surfaces of the reactions of organometallic arene complexes of the type [(η 6-arene)MII(pic)Cl] (where pic = 2-picolinic acid, M = Ru or Os) were examined by a DFT computational study. Among the seven density functional methods, hybrid exchange functional B3LYP outperforms the others to explain the aquation of the complexes. The reactions and binding energies of RuII and OsII arene complexes with both 9EtG and 9EtA were studied to gain insight into the reactivity of these types of organometallic complexes with DNA. The obtained data rationalize experimental observation, contributing to partly understanding the potential biological and medical applications of organometallic complexes.

Reactions of [(η 6-arene)MII(pic)Cl] (M = Ru and Os)





Similar content being viewed by others
References
Reedijk J (2009) Platinum anticancer coordination compounds: study of DNA binding inspires new drug design. Eur J Inorg Chem 2009:1303–1312
Liu HK, Berners-Price SJ, Wang FY, Parkinson JA, Xu JJ, Bella J, Sadler PJ (2006) Diversity in guanine-selective DNA binding modes for an organometallic ruthenium arene complex. Angew Chem Int Ed 45:8153–8156
Liu HK, Wang FY, Parkinson JA, Bella J, Sadler PJ (2006) Ruthenation of duplex and single-stranded d(CGGCCG) by organometallic anticancer complexes. Chem Eur J 12:6151–6165
Dyson PJ (2007) Systematic design of a targeted organometallic antitumor drug in pre-clinical development. Chimia 61:698–703
Sadler PJ, Peacock AFA (2008) Medicinal organometallic chemistry: designing metal arene complexes as anticancer agents. Chem Asian J 3:1890–1899
Schuecker R, John RO, Jakupec MA, Arion VB, Keppler BK (2008) Water-soluble mixed-ligand ruthenium(II) and osmium(II) arene complexes with high antiproliferative activity. Organometallics 27:6587–6595
van Rijt SH, Sadler PJ (2009) Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov Today 14:1089–1097
Levina A, Mitra A, Lay PA (2009) Recent developments in ruthenium anticancer drugs. Metallomics 1:458–470
Pizarro AM, Sadler PJ (2009) Unusual DNA binding modes for metal anticancer complexes. Biochimie 91:1198–1211
Grguric-Sipka S, Stepanenko IN, Lazic JM, Bartel C, Jakupec MA, Arion VB, Keppler BK (2009) Synthesis, X-ray diffraction structure, spectroscopic properties and antiproliferative activity of a novel ruthenium complex with constitutional similarity to cisplatin. Dalton Trans 38:3334–3339
Suss-Fink G (2010) Arene ruthenium complexes as anticancer agents. Dalton Trans 39:1673–1688
Hanif M, Nazarov AA, Hartinger CG, Kandioller W, Jakupec MA, Arion VB, Dyson PJ, Keppler BK (2010) Osmium(II)–versus ruthenium(II)–arene carbohydrate-based anticancer compounds: similarities and differences. Dalton Trans 39:7345–7352
Arndt M, Salih KSM, Fromm A, Goossen LJ, Menges F, Niedner-Schatteburg G (2011) Mechanistic investigation of the Ru-catalyzed hydroamidation of terminal alkynes. J Am Chem Soc 133:7428–7449
Arion VB, Dobrov A, Göschl S, Jakupec MA, Keppler BK, Rapta P (2012) Ruthenium- and osmium-arene-based paullones bearing a TEMPO free-radical unit as potential anticancer drugs. Chem Commun 48:8559–8561
Gligorijević N, Aranđelović S, Filipović L, Jakovljević K, Janković R, Grgurić-Šipka S, Ivanović I, Radulović S, Tešić ŽL (2012) Picolinate ruthenium(II)–arene complex with in vitro antiproliferative and antimetastatic properties: comparison to a series of ruthenium(II)–arene complexes with similar structure. J Inorg Biochem 108:53–61
Kurzwernhart A, Kandioller W, Bächler S, Bartel C, Martic S, Buczkowska M, Mühlgassner G, Jakupec MA, Kraatz H-B, Bednarski PJ et al (2012) Structure–activity relationships of targeted RuII(η 6-p-cymene) anticancer complexes with flavonol-derived ligands. J Med Chem 55:10512–10522
Wu K, Luo Q, Hu W, Li X, Wang F, Xiong S, Sadler PJ (2012) Mechanism of interstrand migration of organoruthenium anticancer complexes within a DNA duplex. Metallomics 4:139–148
Habtemariam A, Melchart M, Fernandez R, Parsons S, Oswald ID, Parkin A, Fabbiani FP, Davidson JE, Dawson A, Aird RE et al (2006) Structure-activity relationships for cytotoxic ruthenium(II) arene complexes containing N, N-, N, O-, and O, O-chelating ligands. J Med Chem 49:6858–6868
Peacock AFA, Habtemariam A, Fernandez R, Walland V, Fabbiani FPA, Parsons S, Aird RE, Jodrell DI, Sadler PJ (2006) Tuning the reactivity of osmium(II) and ruthenium(II) arene complexes under physiological conditions. J Am Chem Soc 128:1739–1748
Peacock AFA, Habtemariam A, Moggach SA, Prescimone A, Parsons S, Sadler PJ (2007) Chloro half-sandwich osmium(II) complexes: influence of chelated N, N-ligands on hydrolysis, guanine binding, and cytotoxicity. Inorg Chem 46:4049–4059
Peacock AFA, Melchart M, Deeth RJ, Habtemariam A, Parsons S, Sadler PJ (2007) Osmium(II) and ruthenium(II) arene maltolato complexes: rapid hydrolysis and nucleobase binding. Chem Eur J 13:2601–2613
Arion VB, Schmid WF, John RO, Jakupec MA, Keppler BK (2007) Highly antiproliferative ruthenium(II) and osmium(II) arene complexes with paullone-derived ligands. Organometallics 26:6643–6652
Peacock AFA, Parsons S, Sadler PJ (2007) Tuning the hydrolytic aqueous chemistry of osmium arene complexes with N, O-chelating ligands to achieve cancer cell cytotoxicity. J Am Chem Soc 129:3348–3357
Kostrhunova H, Florian J, Novakova O, Peacock AFA, Sadler PJ, Brabec V (2008) DNA interactions of monofunctional organometallic osmium(II) antitumor complexes in cell-free media. J Med Chem 51:3635–3643
van Rijt SH, Peacock AFA, Johnstone RDL, Parsons S, Sadler PJ (2009) Organometallic osmium(II) arene anticancer complexes containing picolinate derivatives. Inorg Chem 48:1753–1762
van Rijt SH, Hebden AJ, Amaresekera T, Deeth RJ, Clarkson GJ, Parsons S, McGowan PC, Sadler PJ (2009) Amide linkage isomerism as an activity switch for organometallic osmium and ruthenium anticancer complexes. J Med Chem 52:7753–7764
van Rijt SH, Mukherjee A, Pizarro AM, Sadler PJ (2010) Cytotoxicity, hydrophobicity, uptake, and distribution of osmium(II) anticancer complexes in ovarian cancer cells. J Med Chem 53:840–849
Filak LK, Muhlgassner G, Bacher F, Roller A, Galanski M, Jakupec MA, Keppler BK, Arion VB (2011) Ruthenium- and osmium-arene complexes of 2-substituted indolo[3,2-c]quinolines: synthesis, structure, spectroscopic properties, and antiproliferative activity. Organometallics 30:273–283
van Rijt SH, Kostrhunova H, Brabec V, Sadler PJ (2011) Functionalization of osmium arene anticancer complexes with (poly)arginine: effect on cellular uptake, internalization, and cytotoxicity. Bioconjug Chem 22:218–226
Fu Y, Romero MJ, Habtemariam A, Snowden ME, Song L, Clarkson GJ, Qamar B, Pizarro AM, Unwin PR, Sadler PJ (2012) The contrasting chemical reactivity of potent isoelectronic iminopyridine and azopyridine osmium(ii) arene anticancer complexes. Chem Sci 3:2485
Henkea H, Kandiollera W, Hanifc M, Kepplera BK, Hartinger CG (2012) Organometallic ruthenium and osmium compounds of pyridin-2- and -4-ones as potential anticancer agents. Chem Biodivers 9:1718–1727
Filak LK, Göschl S, Heffeter P, Ghannadzadeh Samper K, Egger AE, Jakupec MA, Keppler BK, Berger W, Arion VB (2013) Metal–arene complexes with indolo[3,2-c]-quinolines: effects of ruthenium vs osmium and modifications of the lactam unit on intermolecular interactions, anticancer activity, cell cycle, and cellular accumulation. Organometallics 32:903–914
Romero-Canelón I, Salassa L, Sadler PJ (2013) The contrasting activity of iodido versus chlorido ruthenium and osmium arene azo- and imino-pyridine anticancer complexes: control of cell selectivity, cross-resistance, p53 dependence, and apoptosis pathway. J Med Chem 56:1291–1300
Wang FY, Habtemariam A, van der Geer EPL, Fernandez R, Melchart M, Deeth RJ, Aird R, Guichard S, Fabbiani FPA, Lozano-Casal P et al (2005) Controlling ligand substitution reactions of organometallic complexes: tuning cancer cell cytotoxicity. Proc Natl Acad Sci USA 102:18269–18274
Dorcier A, Dyson PJ, Gossens C, Rothlisberger U, Scopelliti R, Tavernelli I (2005) Binding of organometallic ruthenium(II) and osmium(II) complexes to an oligonucleotide: a combined mass spectrometric and theoretical study. Organometallics 24:2114–2123
Gossens C, Tavernelli I, Rothlisberger U (2007) Structural and energetic properties of organometallic ruthenium(II) diamine anticancer compounds and their interaction with nucleobases. J Chem Theory Comput 3:1212–1222
Gossens C, Tavernelli I, Rothlisberger U (2009) Binding of organometallic ruthenium(II) anticancer compounds to nucleobases: a computational study. J Phys Chem A 113:11888–11897
Gossens C, Tavernelli I, Rothlisberger U (2008) DNA structural distortions induced by ruthenium-arene anticancer compounds. J Am Chem Soc 130:10921–10928
Gkionis K, Platts JA, Hill JG (2008) Insights into DNA binding of ruthenium arene complexes: role of hydrogen bonding and pi stacking. Inorg Chem 47:3893–3902
Futera Z, Klenko J, Sponer JE, Sponer J, Burda JV (2009) Interactions of the “piano-stool” [ruthenium(II)(η 6-arene)(en)Cl]+ complexes with water and nucleobases; ab initio and DFT study. J Comput Chem 30:1758–1770
Chval Z, Futera Z, Burda JV (2011) Comparison of hydration reactions for “piano-stool” RAPTA-B and [Ru(η6-arene)(en)Cl]+ complexes: density functional theory computational study. J Chem Phys 134:024520
Wang HL, DeYonker NJ, Gao H, Ji LN, Zhao CY, Mao Z-W (2012) Mechanism of aquation and nucleobase binding of ruthenium (II) and osmium (II) arene complexes: a systematic comparison DFT study. J Organomet Chem 704:17–28
Wang HL, DeYonker NJ, Gao H, Tan CP, Zhang XT, Ji LN, Zhao CY, Mao Z-W (2012) Aquation and dimerization of osmium(II) anticancer complexes: a density functional theory study. RSC Adv 2:436–446
Wang HL, DeYonker NJ, Zhang XT, Zhao CY, Ji LN, Mao Z-W (2012) Photodissociation of a ruthenium(II) arene complex and its subsequent interactions with biomolecules: a density functional theory study. J Mol Model 18:4675–4686
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99:2224–2235
Klamt A, Schüürmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perkin Trans 2:799–805
Ehlers W, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler KF, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc-Cu, Y-Ag and La-Au. Chem Phys Lett 208:111–114
Frisch MJ et al (2010) Gaussian 09, revision A.01. Gaussian, Wallingford
Wang F, Chen HM, Parsons S, Oswald LDH, Davidson JE, Sadler PJ (2003) Kinetics of aquation and anation of ruthenium(II) arene anticancer complexes, acidity and X-ray structures of aqua adducts. Chem Eur J 9:5810–5820
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. Phys Chem 98:11623–11627
Deubel DV, Lau JKC (2006) In silico evolution of substrate selectivity: comparison of organometallic ruthenium complexes with the anticancer drug cisplatin. Chem Commun 2006:2451–2453
Chen HM, Parkinson JA, Morris RE, Sadler PJ (2003) Highly selective binding of organometallic ruthenium ethylenediamine complexes to nucleic acids: novel recognition mechanisms. J Am Chem Soc 125:173–186
Chen HM, Parkinson JA, Novakova O, Bella J, Wang FY, Dawson A, Gould R, Parsons S, Brabec V, Sadler PJ (2003) Induced-fit recognition of DNA by organometallic complexes with dynamic stereogenic centers. Proc Natl Acad Sci USA 100:14623–14628
Chen HM, Parkinson JA, Parsons S, Coxall RA, Gould RO, Sadler PJ (2002) Organometallic ruthenium(II) diamine anticancer complexes: arene-nucleobase stacking and stereospecific hydrogen-bonding in guanine adducts. J Am Chem Soc 124:3064–3082
Baik MH, Friesner RA, Lippard SJ (2003) Theoretical study of cisplatin binding to purine bases: why does cisplatin prefer guanine over adenine. J Am Chem Soc 125:14082–14092
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos., 21173273) and Dr. Start Fund of Guangdong University of Petrochemical Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 169 kb)
Appendix
Appendix
Abbreviations
Acronym | Chemical Formula |
|---|---|
pic | 2-picolinic acid |
p-cym | p-cymene |
bip | biphenyl |
bz | benzene |
9EtG | 9-ethyl guanine |
9EtA | 9-ethyl adenine |
Ru-H2O | [(η 6-bz)RuII(pic)(H2O)]2+ |
bz-Ru-A | [(η 6-bz)RuII(pic)(9EtA)]2+ |
bz-Os-A | [(η 6-bz)OsII(pic)(9EtA)]2+ |
cym-Ru | [(η 6-p-cym)RuII(pic)Cl]+ |
cym-Os | [(η 6-p-cym)OsII(pic)Cl]+ |
bip-Ru | [(η 6-bip)RuII(pic)Cl]+ |
bip-Os | [(η 6-bip)OsII(pic)Cl]+ |
bz-Ru | [(η 6-bz)RuII(pic)Cl]+ |
bz-Os | [(η 6-bz)OsII(pic)Cl]+ |
Os-H2O | [(η 6-bz)OsII(pic)(H2O)] 2+ |
bz-Ru-G | [(η 6-bz)RuII(pic)(9EtG)]2+ |
bz-Os-G | [(η 6-bz)OsII(pic)(9EtG)]2+ |
Rights and permissions
About this article
Cite this article
Wang, H., Zeng, X., Zhou, R. et al. A comparative DFT study on aquation and nucleobase binding of ruthenium (II) and osmium (II) arene complexes. J Mol Model 19, 4849–4856 (2013). https://doi.org/10.1007/s00894-013-1987-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1987-5