Abstract
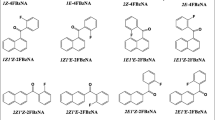
The reaction pathways of several Friedel–Crafts acylations involving phenyl aromatic compounds were studied using density functional theory. The reactions were related to the Friedel–Crafts polycondensation of polyaryletherketones. In particular, the acylation of benzene with benzoyl chloride to form benzophenone and variations on this reaction were investigated. The acylation of benzene by one molecule of terephthaloyl chloride or isophthaloyl chloride as well as acylations at the m-, o-, and p-positions of diphenyl ether with one molecule of benzoyl chloride were studied. Adding an additional acyl chloride group to the electrophile appeared to have little influence on the reaction pathway, although the activation energy for the C–C bond-forming steps that occurred when isophthaloyl choride was used was different to the activation energy observed when terephthaloyl chloride was used. Upon changing the nucleophile to diphenyl ether, the reactivity changed according to the trend predicted on based on the o-, p-directing effects of the ether group. The deprotonation step that restored aromaticity varied widely according to the reaction. The rate-determining step in all of the studied reactions was the formation of the acylium ion, followed in importance by either the formation of the Wheland intermediate or the abstraction of hydrogen, depending on the reactivity of the nucleophile.








Similar content being viewed by others
References
Carey FA, Sundberg RJ (2007) Advanced organic chemistry, part A: structure and mechanisms, 5th edn. Springer, New York
Friedel C, Crafts JM (1877) Compt Rend 84:1392–1395, 1450–1454
Olah GA (1963) Friedel–Crafts and related reactions, vol III, acylation and related reactions. Interscience, New York
Eyley SC (1991) Comp Org Synth 2:707–731
Heaney H (1991) Comp Org Synth 2:733–752
Cassimatis D, Bonnin JP, Theophanides T (1970) Can J Chem 48:3860–3871. doi:10.1139/v70-650
Csihony S, Mehdi H, Homonnay Z, Vértes A, Farkas Ö, Horváth IT (2002) J Chem Soc Dalton Trans 680–685. doi:10.1039/B109303G
Xu T, Barich DH, Torres PD, Nicholas JB, Haw JF (1997) J Am Chem Soc 119:396–405. doi:10.1021/ja962944n
Olah GA (1971) Acc Chem Res 4:240–248. doi:10.1021/ar50043a002
Olah GA, Kobayashi S, Tashiro M (1972) J Am Chem Soc 94:7448–7461. doi:10.1021/ja00776a030
Boer FP (1968) J Am Chem Soc 94:6706–6717. doi:10.1021/ja01026a025
Chevrier B, Weiss R (1974) Angew Chem 86:12–21. doi:10.1002/ange.19740860103
Tarakeshwar P, Lee JY, Kim KS (1998) J Phys Chem A 102:2253–2255. doi:10.1021/jp9807322
Tarakeshwar P, Kim KS (1999) J Phys Chem A 103:9116–9124. doi:10.1021/jp992019y
Jasien PG (1995) J Phys Chem 99:6502–6508. doi:10.1021/j100017a034
Volkov AN, Timoshkin AY, Suvorov AV (2004) Int J Quantum Chem 100:412–418. doi:10.1002/qua.20182
Volkov AN, Timoshkin AY, Suvorov AV (2005) Int J Quantum Chem 104:256–260. doi:10.1002/qua.20420
Chattaraj PK, Sarkar U, Elango M, Parthasarathi R, Subramanian V (2005) Electrophilicity as a possible descriptor of the kinetic behavior. http://arxiv.org/abs/physics/0509089. Accessed 18 Jan 2013
Osamura Y, Terada K, Kobayashi Y, Okazaki R, Ishiyama Y (1999) J Mol Struct THEOCHEM 461–462:399–416. doi:10.1016/S0166-1280(98)00452-7
Gothelf AS, Hansen T, Jørgensen KA (2001) J Chem Soc Perkin Trans 1:854–860. doi:10.1039/B009669P
Olah GA, Török B, Joschek JP, Bucsi I, Esteves PM, Rasul G, Prakash GKS (2002) J Am Chem Soc 124:11379–11391. doi:10.1021/ja020787o
Yamabe S, Yamazaki S (2009) J Phys Org Chem 22:1094–1103. doi:10.1002/poc.1564
Olah GA, Moffatt ME, Kuhn SJ, Hardie BA (1964) J Am Chem Soc 86:2198–2202. doi:10.1021/ja01065a019
Olah GA, Lukas J, Lukas E (1969) J Am Chem Soc 91:5319–5323. doi:10.1021/ja01047a021
Olah GA, Kobayashi S (1971) J Am Chem Soc 93:6964–6967. doi:10.1021/ja00754a045
Effenberger F, Maier AH (2001) J Am Chem Soc 123:3429–3433. doi:10.1021/ja0022066
Olah GA, Kuhn SJ, Flood SH, Hardie BA (1964) J Am Chem Soc 86:2203–2209. doi:10.1021/ja01065a020
Dowdy D, Gore PH, Waters DN (1991) J Chem Soc Perkin Trans 2:1149–1159. doi:10.1039/P29910001149
Ehrenson S, Brownlee RTC, Taft RW (1973) A generalized treatment of substituent effects in the benzene series. A statistical analysis by the dual substituent parameter equation. In: Streitweiser A Jr, Taft RW (eds) Progress in physical organic chemistry, vol 10. Wiley, New York, pp 1–80
Rosato Dominick V, Rosato Donald V, Rosato MV (2004) Plastic product material and process selection handbook. Elsevier, Oxford
Salamone JC (ed) (1999) Concise polymeric materials encyclopedia. CRC, Boca Raton
Johnson RN, Farnham AG, Clendinning RA, Hale WF, Merriam CN (1967) J Polym Sci A-1 5:2375–2398. doi:10.1002/pol.1967.150050916
Attwood TE, Dawson PC, Freeman JL, Hoy LR, Rose JB, Staniland PA (1981) Polymer 22:1096–1103. doi:10.1016/0032-3861(81)90299-8
Rose JB, Staniland PA (1982) Thermoplastic aromatic polyetherketones. US Patent 4,320,224
Fukawa I, Tanabe T, Dozono T (1991) Macromolecules 24:3838–3844. doi:10.1021/ma00013a016
Bonner WH (1962) Aromatic polyketones and preparation thereof. US Patent 3,065,205
Goodman I, McIntyre JE, Russel W (1964) Process for the preparation of polymeric ketones. Brit Patent 971,227; Chem Abstr (1964) 61:14805b
Brugel E (1991) Stabilization of poly(ether ketone ketones). US Patent 4,987,171
Gay FP, Brunette CM (1989) Ordered polyetherketones, US Patent 4,816,556
Rueda DR, Zolotukhin MG, Cagiao ME, Ania F, Dosière M, Villers D, de Abajo J (2001) J Macromol Sci B Phys 40:709–731. doi:10.1081/MB-100107557
Ueda M, Sato M (1987) Macromolecules 20:2675–2678. doi:10.1021/ma00177a007
Zolotukhin MG, Rueda DR, Bruix M, Cagiao ME, Balta Calleja FJ, Bulai A, Gileva NG, Van der Elst L (1997) Polymer 38:3441–3454. doi:10.1016/S0032-3861(96)00909-3
Frisch MJ et al. (2009) GAUSSIAN 09. Gaussian, Inc., Wallingford
Chai JD, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615–6620. doi:10.1039/B810189B
Chai JD, Head-Gordon M (2008) J Chem Phys 128:084106–084121. doi:10.1063/1.2834918
Becke AD (1993) J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Stephens PJ, Devlin FJ, Chabalowski MJ, Frisch MJ (1994) J Phys Chem 98:11623–11627. doi:10.1021/j100096a001
Burke KJ (2012) Chem Phys 136:150901–150908. doi:10.1063/1.4704546
Tsuzuki S, Luthi HP (2001) J Chem Phys 114:3949–3957. doi:10.1063/1.1344891
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918. doi:10.1021/jp048147q
Zhao Y, Truhlar DG (2005) J Chem Theory Comput 1:415–432. doi:10.1021/ct049851d
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3094. doi:10.1021/cr9904009
Cancès E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041. doi:10.1063/1.474659
Reed AE, Weinstock RB, Weinhold F (1984) J Chem Phys 83:735–746. doi:10.1063/1.449486
Politzer P, Mulliken RS (1971) J Chem Phys 55:5135–5137. doi:10.1063/1.1675638
Fukui K (1981) Acc Chem Res 14:363–368. doi:10.1021/ar00072a001
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2164. doi:10.1063/1.456010
Braga AAC, Ujaque G, Maseras F (2006) Organometallics 25:3647–3658. doi:10.1021/om060380i
Fukuzumi S, Kochi JK (1982) J Am Chem Soc 104:7599–7609
Boys SF, Bernardi F (1970) Mol Phys 19:553–566. doi:10.1080/00268977000101561
McDaniel DH, Brown HC (1958) J Org Chem 23:420–427. doi:10.1021/jo01097a026
Stock LM, Brown HC (1963) Adv Phys Org Chem 1:35–154. doi:10.1016/S0065-3160(08)60277-4
Brătulescu G (2003) Rev Roum Chim 48:175–177
Sawant DP, Devassy BM, Halligudi SB (2004) J Mol Catal Chem 217:211–217. doi:10.1016/j.molcata.2004.03.038
Pitoňák M, Neogrády P, Řezáč J, Jurečka P, Urban M, Hobza P (2008) J Chem Theory Comput 4:1829–1834. doi:10.1021/ct800229h
Cooper VR (2010) Phys Rev B 81:161104
Zielinski F, Tognetti V, Joubert L (2012) Chem Phys Lett 27:67–72. doi:10.1016/j.cplett.2012.01.011
Parr RG, Yang W (1984) J Am Chem Soc 106:4049–4050. doi:10.1021/ja00326a036
Acknowledgments
The authors would like to acknowledge the Centre de Resources Informatique de Haute-Normandie (CRIHAN), and in particular Dr. P. Bousquet-Melou and Mr. H. Prigent. The authors would like to thank the European Regional Development Fund (ERDF), the Conventions Industrielles de Formation pour la Recherche (CIFRE), the Region Haute-Normandie, and the Arkema group that funded this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melissen, S.T.A.G., Tognetti, V., Dupas, G. et al. A DFT study of the Al2Cl6-catalyzed Friedel–Crafts acylation of phenyl aromatic compounds. J Mol Model 19, 4947–4958 (2013). https://doi.org/10.1007/s00894-013-1984-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1984-8