Abstract
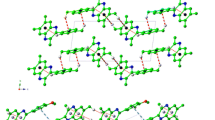
The single crystal architecture of the high-symmetry octathio[8]circulene and sym-tetraselenatetrathio[8]circulene is studied at the density functional theory (DFT) level with the quantum theory of atoms in molecules (QTAIMs) approach to the electron density distribution analysis. The presence of stabilizing intermolecular C---C, C---S and C---Se contacts in the longitudinal and transversal projections of the single crystals is postulated on the grounds of the previous high-resolution X-ray data for octathio[8]circulene; it is supported by the present QTAIM calculations and also predicted in some new details for both circulenes. We suggest that the appearance of the observed red color for the monocrystalline octathio[8]circulene is caused by strong intermolecular interactions between the molecules in the single crystal. However, the intermolecular interactions for the sym-tetraselenatetrathio[8]circulene crystal fragment are weaker and molecular layers are more friable in comparison to octathio[8]circulene crystal structure. These lead to the absence of visible absorption for the sym-tetraselenatetrathio[8]circulene crystal.

3D architecture of molecular crystals for two studied circulenes






Similar content being viewed by others
References
Chernichenko KY, Sumerin VV, Shpanchenko RV, Balenkova ES, Nenajdenko VG (2006) “Sulflower”: a new form of carbon sulfide. Angew Chem Int Ed 118:7527–7530
Ivasenko O, MacLeod JM, Chernichenko KYu, Balenkova ES, Shpanchenko RV, Nenajdenko VG, Rosei F, Perepichka DF (2009) Supramolecular assembly of heterocirculenes in 2D and 3D. Chem Commun doi: 10.1039/b819532c
Dadvand A, Cicoira F, Chernichenko KYu, Balenkova ES, Osuna RM, Rosei F, Nenajdenko VG, Perepichka DF (2008) Heterocirculenes as a new class of organic semiconductors. Chem Commun doi: 10.1039/b809259a
Chernichenko KY, Balenkova ES, Nenajdenko VG (2008) From thiophene to sulflower. Mendeleev Commun 18:171–179
Erdtman H, Hogberg HE (1968) Tetranaphthocyclo-octatetraene tetra-oxide, a cyclisation product from α-naphthoquinone. Chem Commun (London) pp 773–774
Nielsen CB, Brock-Nannestad T, Reenberg TK, Hammershøj P, Christensen JB, Stouwdam JW, Pittelkow M (2010) Organic light-emitting diodes from symmetrical and unsymmetrical π-extended tetraoxa[8]circulene. Chem Eur J 16:13030–13034
Nielsen CB, Brock-Nannestad T, Hammershøj P, Reenberg TK, Schau-Magnussen M, Trpcevski D, Salcedo R, Baryshnikov GV, Minaev BF, Pittelkow M (2013) Azatrioxa[8]circulenes: planar anti-aromatic cyclooctatetraenes. Chem Eur J 19:3898–3904
Baryshnikov GV, Minaev BF, Minaeva VA, Baryshnikova AT, Pittelkow M (2012) DFT and QTAIM study of the tetra-tert-butyltetraoxa[8]circulene regioisomers structure. J Mol Struct 1026:127–132
Minaeva VA, Minaev BF, Baryshnikov GV, Agren H, Pittelkow M (2012) Experimental and theoretical study of IR and Raman spectra of tetraoxa[8]circulenes. Vib Spectrosc 61:156–166
Minaeva VA, Minaev BF, Baryshnikov GV, Romeyko OM, Pittelkow M (2013) The FTIR spectra of substituted tetraoxa[8]circulenes and their assignments based on DFT calculations. Vib Spectrosc 65:147–158
Baryshnikov GV, Minaev BF, Pittelkow M, Nielsen CB, Salcedo R (2013) Nucleus-independent chemical shifts criterion of aromaticity in the π-extended tetraoxa[8]circulenes. J Mol Model 19:847–850
Minaev BF, Baryshnikov GV, Minaeva VA (2011) Density functional theory study of electronic structure and spectra of tetraoxa[8]circulenes. Comput Theor Chem 972:68–74
Minaeva VA, Minaev BF, Baryshnikov GV, Romeyko ON, Pittelkow M (2012) Raman spectra of tetraoxa[8]circulenes. p-dinaphthalenodiphenylenotetrafuran and its tetraalkyl derivatives (DFT study and experiment). J Appl Spectrosc 79:695–707
Takuya F, Michio MM, Awaga K (2009) Electrochemical field-effect transistors of octathio[8]circulene robust thin films with ionic liquids. Chem Phys Lett 483:81–83
Fujimoto T, Matsushita MM, Yoshikawa H, Awaga K (2008) Electrochemical and electrochromic properties of octathio[8]circulene thin films in ionic liquids. J Am Chem Soc 130:15790–15791
Gahungu G, Zhang J (2008) Shedding light on octathio[8]circulene and some of its plate-like derivatives. Phys Chem Chem Phys 10:1743–1747
Mohakud S, Pati SK (2009) Large carrier mobilities in octathio[8]circulene crystals: a theoretical study. J Mater Chem 19:4356–4361
Tang X-D, Liao Y, Gao H-Z, Geng Y, Su Z-M (2012) Theoretical study of the bridging effect on the charge carrier transport properties of cyclooctatetrathiophene and its derivatives. J Mater Chem 22:6907–6918
Fujimoto T, Matsushita MM, Awaga K (2010) Dual-gate field-effect transistors of octathio[8]circulene thin-films with ionic liquid and SiO2 gate dielectrics. Appl Phys Lett 97:123303
Bukalov SS, Leites LA, Lyssenko KA, Aysin RR, Korlyukov AA, Zubavichus JV, Chernichenko KY, Balenkova ES, Nenajdenko VG, Antipin MY (2008) Two modifications formed by “sulflower” C16S8 molecules, their study by XRD and optical spectroscopy (Raman, IR, UV–vis) methods. J Phys Chem A 112:10949–10961
Fujimoto T, Suizu R, Yoshikawa H, Awaga K (2008) Molecular, crystal, and thin-film structures of octathio[8]circulene: release of antiaromatic molecular distortion and lamellar structure of self-assembling thin films. Chem Eur J 14:6053–6056
Aragó J, Viruela PM, Ortí E (2009) From linear quaterthiophene to sulflower: a comparative theoretical study. J Mol Struct (THEOCHEM) 912:27–31
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 7:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Francl MM, Petro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization type basis set for second-row elements. J Chem Phys 77:3654–3665
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Montgomery J, Vreven J, Kudin K, Burant J, Millam J, Iyengar S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox RJ, Hratchian H, Cross J, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R, Yazyev O, Austin A, Cammi R, Pomelli C, Ochterski J, Ayala P, Morokuma K, Voth G, Salvador P, Dannenberg J, Zakrzewski V, Dapprich S, Daniels A, Strain M, Farkas O, Malick D, Rabuck A, Raghavachari K, Foresman J, Ortiz J, Cui Q, Baboul A, Clifford S, Cioslowski J, Stefanov B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R, Fox D, Keith T, Al-Laham M, Peng C, Nanayakkara A, Challacombe M, Gill P, Johnson B, Chen W, Wong M, Gonzalez C, Pople J (2004) Gaussian 03, revision C.02. Gaussian, Inc, Wallingford, CT
Bader RFW (1990) Atoms in molecules. A quantum theory. Clarendon, Oxford, U.K
Bader RFW, Essen H (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960
Cremer D, Kraka E (1984) Description of the chemical bond in terms of local properties of electron density and energy. Croat Chem Acta 57:1259–1281
Bader RFW, See TS, Cremer D, Kraka E (1983) Description of conjugation and hyperconjugation in terms of electron distributions. J Am Chem Soc 105:5061–5068
Keith TA (2010) AIMAll (Version 10.07.25), TK Gristmill Software. Overland Park KS, USA, www.aim.tkgristmill.com
Bertolotti F, Forni A, Gervasio G, Marabello D, Diana E (2012) Experimental and theoretical charge density of hydrated cupric acetate. Polyhedron 42:118–127
Gall V, Breza M (2009) QTAIM study of transition metal complexes with cyclophosphazene-based multisite ligands I: Zinc(II) and Nickel(II) complexes. Polyhedron 28:521–524
Rybalova TV, Bagryanskaya IY (2009) C-F…π, F…H, and F…F intermolecular interactions and F-aggregation: role in crystal engineering of fluoroorganic compounds. J Struct Chem 50:741–753
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Espinosa E, Alkorta I, Rozas I, Elguero J, Molins E (2001) About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem Phys Lett 336:457–461
Latosińska N, Latosińska M, Tomczak MA, Seliger J, Žagar V (2011) Supramolecular synthon pattern in solid clioquinol and cloxiquine (APIs of antibacterial, antifungal, antiaging and antituberculosis drugs) studied by 35Cl NQR, 1H-17O and 1H-14N NQDR and DFT/QTAIM. J Mol Model 17:1781–1800
Nguyen TH, Groundwater PW, Platts JA, Hibbs DE (2012) Experimental and theoretical charge density studies of 8-hydroxyquinoline cocrystallized with salicylic acid. J Phys Chem A 116:3420–3427
Shishkina AV, Zhurov VV, Stash AI, Vener MV, Pinkerton AA, Tsirelson VG (2013) Noncovalent interactions in crystalline picolinic acid N-oxide: insights from experimental and theoretical charge density analysis. Cryst Growth Des 13:816–828
Runge E, Gross EKU (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52:997–1000
González Moa MJ, Mandado M, Mosquera RA (2007) A computational study on the stacking interaction in quinhydrone. J Phys Chem A 111:1998–2001
Abramov YA (2007) On the possibility of kinetic energy density evaluation from the experimental electron-density distribution. Acta Crystallogr A 53:264–272
Minaev BF (2007) Electronic mechanisms of molecular oxygen activation. Russ Chem Rev 76:989–1011
Minaev BF (2005) Intensity of singlet–triplet transitions in C60 fullerene calculated on the basis of the time-dependent density functional theory and taking into account the quadratic response. Opt Spectrosc 98:336–340
Minaev BF, Minaev AB (2005) Calculation of the phosphorescence of porphyrins by the density functional method. Opt Spectrosc 98:214–219.
Minaev BF (2004) Ab initio study of the ground state properties of molecular oxygen. Spectrochim Acta A 60:1027–1041
Jahn HA, Teller E (1937) Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc Roy Soc 161:220–235
Rubio-Pons O, Loboda O, Minaev B, Schimmelpfennig B, Vahtras O, Ågren H (2003) CASSCF calculations of triplet-state properties. Applications to benzene derivatives. Mol Phys 101:2103–2114
Minaev BF, Minaeva VA (2001) MCSCF response calculations of the excited states properties of the O2 moelcule and a part of its spectrum. Phys Chem Chem Phys 3:720–729
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baryshnikov, G.V., Minaev, B.F., Minaeva, V.A. et al. Single crystal architecture and absorption spectra of octathio[8]circulene and sym-tetraselenatetrathio[8]circulene: QTAIM and TD-DFT approach. J Mol Model 19, 4511–4519 (2013). https://doi.org/10.1007/s00894-013-1962-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1962-1