Abstract
The applications of endohedral non-metallic fullerenes are limited by their low production rate. Recently, an explosive method developed in our group shows promise to prepare He@C60 at fairly high yield, but the mechanism of He inserting into C60 cage at explosive conditions was not clear. Here, ab initio molecular dynamics analysis has been used to simulate the collision between C60 molecules at high-temperature and high-pressure induced by explosion. The results show that defects formed on the fullerene cage by collidsion can effectively decrease the reaction barrier for the insertion of He into C60, and the self-healing capability of the defects was also observed.

Simulation of He@C60 formation by explosive method. Ab initio molecular dynamics has been used to simulate collision of C60. Defects caused by fullerenes reaction in explosion are shown by theory. The defects decrease the reaction barrier for He inserting into C60 cage. The method provides a promising technique to synthesized He@C60



Similar content being viewed by others
References
Ball P (2011) Material witness: caged water. Nat Mater 10:649–649. doi:10.1038/nmat3107
Ju CY, Suter D, Du JF (2007) Two-qubit gates between noninteracting qubits in endohedral-fullerene-based quantum computation. Phys Rev A 75:12318–12322
Cimpoesu F, Ito S, Shimotani H, Takagi H, Dragoe N (2011) Vibrational properties of noble gas endohedral fullerenes. Phys Chem Chem Phys 13:9609–9615
Saunders M, Jiménez-Vázquez HA, Cross RJ, Mroczkowski S, Freedberg DI, Anet FAL (1994) Probing the interior of fullerenes by 3He NMR spectroscopy of endohedral 3He@C60 and 3He@C70. Nature 367:256–258
Buhl M, Patchkovskii S, Thiel W (1997) Interaction energies and NMR chemical shifts of noble gases in C60. Chem Phys Lett 275:14–18
Dodziuk H, Dolgonos G, Lukin O (2001) Molecular mechanics study of endohedral fullerene complexes with small molecules. Carbon 39:1907–1911
Kareev IE, Bubnov VP, Laukhina EE, Koltover VK, Yagubskii EB (2003) Endohedral metallofullerenes M@C82 (M=La, Y): synthesis and transport properties. Carbon 41:1375–1380
Saunders M, Jiménez-Vázquez HA, Cross RJ, Mroczkowski S, Gross ML, Giblin DE, Poreda RJ (1994) Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high-pressure. J Am Chem Soc 116:2193–2194
Saunders M, Cross RJ, Jiménez-Vázquez HA, Shimshi R, Khong A (1996) Noble gas atoms inside fullerenes. Science 271:1693–1697
Murata Y, Murata M, Komatsu K (2003) 100 % encapsulation of a hydrogen molecule into an open-cage fullerene derivative and gas-phase generation of H2@C60. J Am Chem Soc 125:7152–7153
Stanisky CM, Cross RJ, Saunders M, Murata M, Murata Y, Komatsu K (2005) Helium entry and escape through a chemically opened window in a fullerene. J Am Chem Soc 127:299–302
Komatsu K, Murata M, Murata Y (2005) Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 307:238–240
Stanisky CM, Cross RJ, Saunders M (2009) Putting atoms and molecules into chemically opened fullerenes. J Am Chem Soc 131:3392–3395
Balch AL (2011) H2O in a desert of carbon atoms. Science 333:531–532
Kurotobi K, Murata Y (2011) A single molecule of water encapsulated in fullerene C60. Science 333:613–616
Teligmann R, Krawez N, Lin S, Hertel IV, Campbell EEP (1996) Endohedral fullerene production. Nature 382:407–408
Shimshi R, Cross RJ, Saunders M (1997) Beam implantation: a new method for preparing cage molecules containing atoms at high incorporation levels. J Am Chem Soc 119:1163–1164
Ohtsuki T, Ohno K, Shiga K, Kawazoe Y, Maruyama Y, Masumoto K (1998) Insertion of Xe and Kr atoms into C60 and C70 fullerenes and the formation of dimers. Phys Rev Lett 81:967–970
Patchkovskii S, Thiel W (1996) How does helium get into buckminsterfullerene? J Am Chem Soc 118:7164–7172
Zahn D (2005) Unprejudiced identification of reaction mechanisms from biased transition path sampling. J Chem Phys 123:44104–44110
Granot R, Baer R (2008) A tight-binding potential for helium in carbon systems. J Chem Phys 129:214102–214106
Peng RF, Chu SJ, Huang YM, Yu HJ, Wang TS, Jin B et al (2009) Preparation of He@C60 and He2@C60 by an explosive method. J Mater Chem 19:3602–3605
Manaa MR, Fried LE, Melius CF, Elstner M, Frauenheim T (2002) Decomposition of HMX at extreme conditions a molecular dynamics simulation. J Am Chem Soc 106:9024–9029
Dlott DD (1999) Ultrafast spectroscopy of shock waves in molecular materials. Annu Rev Phys Chem 50:251–278
Strachan A, van Duin ACT, Chakraborty D, Dasgupta S, Goddard WA III (2003) Shock waves in high-energy materials: the initial chemical events in nitramine RDX. Phys Rev Lett 91:98301–98304
Strachana A, Kober EM, van Duin ACT, Oxgaard J, Goddard WA III (2005) Thermal decomposition of RDX from reactive molecular dynamics. J Chem Phys 122:54502–54511
Liu L, Krack M, Michaelides A (2008) Density oscillations in a nanoscale water film on salt: insight from Ab initio molecular dynamics. J Am Chem Soc 130:8572–8573
Liu LM, Krack M, Michaelides A (2009) Interfacial water: a first principles molecular dynamics study of a nanoscale water film on salt. J Chem Phys 130:234702–234713
Vondele JV, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput Phys Commun 167:103–128
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Grimme S, Antony J, Ehrlich S, Krieget H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154122
Henkelman G, Uberuaga BP, Jonsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901–9904
Kabir M, Mukherjee S, Saha-Dasgupta T (2011) Substantial reduction of Stone-Wales activation barrier in fullerene. Phys Rev B 84:205404–205410
Hesselmann A, Korona T (2011) On the accuracy of DFT-SAPT, MP2, SCS-MP2, MP2C, and DFT+Disp methods for the interaction energies of endohedral complexes of the C60 fullerene with a rare gas atom. Phys Chem Chem Phys 13:732–743
Cao BP, Peres T, Cross RJ, Saunders M, Lifshitz C (2005) Unimolecular dissociations of C70 + and its noble gas endohedral cations Ne@C70 + and Ar@C70 +: Cage-binding energies for C2 loss. J Phys Chem A 109:10257–10263
Cao B, Peres T, Cross RJ, Saunders M, Lifshitz C (2001) Do nitrogen-atom- containing endohedral fullerenes undergo the shrink-wrap mechanism? J Phys Chem A 105:2142–2146
Jakowski J, Irle S, Morokuma K (2010) Collision-induced fusion of two C60 fullerenes: quantum chemical molecular dynamics simulations. Phys Rev B 82:125443–125450
Brink C, Hvelplund P, Shen H, Jiménez-Vázquez HA, Cross RJ, Saunders M (1998) Collisional fragmentation of Ar@C60. Chem Phys Lett 286:28–34
Saunders M, Jiménez-Vázquez HA, Cross RJ, Poredaet RJ (1993) Stable compounds of helium and neon: He@C60 and Ne@C60. Science 259:1428–1430
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (Project NOs. 50972122, 11244001, 51222212), Youth Innovation Research Team of Sichuan for Carbon Nanomaterials (Project No. 2011JTD0017), state key laboratory cultivation base for nonmetal composites and functional materials, Southwest University of Science and Technology (Project NO. 11ZXFK16) and Science Foundation of China Academy of Engineering Physics (Project NO. 11ZH0157).
Author information
Authors and Affiliations
Corresponding author
Additional information
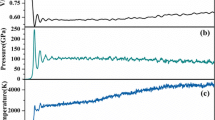
To experimentally study explosion shock wave, a method of small scale gap test was improved to guarantee the steady propagation of shock wave, a Mn-Cu manometer was used to measure the detonation pressure (23.5–28.8 GPa), and the range of detonation velocity was 2 to 5 × 103 m s-1. It was found that there is a close relation between explosion shock wave of explosive quality and the yields of He@C60
Rights and permissions
About this article
Cite this article
Li, JY., Liu, LM., Jin, B. et al. Ab initio molecular dynamics simulation on the formation process of He@C60 synthesized by explosion. J Mol Model 19, 1705–1710 (2013). https://doi.org/10.1007/s00894-012-1737-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1737-0