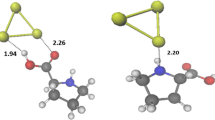
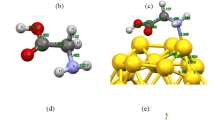
Abstract
Interaction between metal nanoparticles and biomolecules is important from the view point of developing and designing biosensors. Studies on proline tagged with gold nanoclusters are reported here using density functional theory (DFT) calculations for its structural, electronic and bonding properties. Geometries of the complexes are optimized using the PBE1PBE functional and mixed basis set, i. e., 6-311++G for the amino acid and SDD for the gold clusters. Equilibrium configurations are analyzed in terms of interaction energies, molecular orbitals and charge density. The complexes associated with cluster composed of an odd number of Au atoms show higher stability. Marked decrease in the HOMO-LUMO gaps is observed on complexation. Major components of interaction between the two moieties are: the anchoring N-Au and O-Au bond; and the non covalent interactions between Au and N-H or O-H bonds. The electron affinities and vertical ionization potentials for all complexes are calculated. They show an increased value of electron affinity and ionization potential on complexation. Natural bond orbital (NBO) analysis reveals a charge transfer between the donor (proline) and acceptor (gold cluster). The results indicate that the nature of interaction between the two moieties is partially covalent. Our results will be useful for further experimental studies and may be important for future applications.






Similar content being viewed by others
References
Parker JF, Fields-Zinna CA, Murray RW (2010) The story of a monodisperse gold nanoparticle: Au25l18. Acc Chem Res 43(9):1289–1296
Li J, Li X, Zhai HJ, Wang LS (2003) Au20: a tetrahedral cluster. Science 299(5608):864–867
Woehrle GH, Warner MG, Hutchison JE (2002) Ligand exchange reactions yield subnanometer, thiol-stabilized gold particles with defined optical transitions. J Phys Chem B 106(39):9979–9981
Whetten RL, Shafigullin MN, Khoury JT, Schaaff TG, Vezmar I, Alvarez MM, Wilkinson A (1999) Crystal structures of molecular gold nanocrystal arrays. Acc Chem Res 32(5):397–406
Jin R, Cao Y, Mirkin CA, Kelly KL, Schatz GC, Zheng JG (2001) Photoinduced conversion of silver nanospheres to nanoprisms. Science 294(5548):1901–1903
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592):607–609
Chen XJ, Sanchez-Gaytan BL, Qian Z, Park SJ (2012) Noble metal nanoparticles in DNA detection and delivery. Nanomed Nanobiotech 4(3):273–290
Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ (1999) Infrared extinction properties of gold nanoshells. Appl Phys Lett 75(19):2897–2899
Shafai GS, Shetty S, Krishnamurty S, Shah V, Kanhere DG (2007) Density functional investigation of the interaction of acetone with small gold clusters. J Chem Phys 126(1):014704
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem Lett 16(2):405–408
Lee TH, Ervin KM (1994) Reactions of copper group cluster anions with oxygen and carbon monoxide. J Phys Chem 98(40):10,023–10,031
Garzón IL, Rovira C, Michaelian K, Beltrán MR, Ordejón P, Junquera J, Sánchez-Portal D, Artacho E, Soler JM (2000) Do thiols merely passivate gold nanoclusters? Phys Rev Lett 85:5250–5251
Häkkinen H, Moseler M, Landman U (2002) Bonding in Cu, Ag, and Au clusters: relativistic effects, trends, and surprises. Phys Rev Lett 89:033,401–033,404
Furche F, Ahlrichs R, Weis P, Jacob C, Gilb S, Bierweiler T, Kappes MM (2002) The structures of small gold cluster anions as determined by a combination of ion mobility measurements and density functional calculations. J Chem Phys 117(15):6982–6990
Pyykkö P (1988) Relativistic effects in structural chemistry. Chem Rev 88(3):563–594
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chem Int Ed 43(34):4412–4456
Huang W, Wang LS (2009) Probing the 2d to 3d structural transition in gold cluster anions using argon tagging. Phys Rev Lett 102:153,401–153,404
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2007) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769
Chen P, Mwakwari S, Oyelere A (2008) Gold nanoparticles: from nanomedicine to nanosensing. Nanotech Sci Apps 1:45–66
De M, You CC, Srivastava S, Rotello VM (2007) Biomimetic interactions of proteins with functionalized nanoparticles: a thermodynamic study. J Am Chem Soc 129(35):10,747–10,753
Yu J, Choi S, Richards CI, Antoku Y, Dickson RM (2008) Live cell surface labeling with fluorescent ag nanocluster conjugatesâ€. Photochem Photobiol 84(6):1435–1439
Upert G, Bouillére F, Wennemers H (2012) Oligoprolines as scaffolds for the formation of silver nanoparticles in defined sizes: Correlating molecular and nanoscopic dimensions. Angew Chem Int Ed 51(17):4231–4234
Tabarin T, Kulesza A, Antoine R, Mitrić R, Broyer M, Dugourd P, Bonačić Koutecký V (2008) Absorption enhancement and conformational control of peptides by small silver clusters. Phys Rev Lett 101:213001–213005
Bonačić Koutecký V, Kulesza A, Gell L, Mitrić R, Antoine R, Bertorelle F, Hamouda R, Rayane D, Broyer M, Tabarin T, Dugourd P (2012) Silver cluster-biomolecule hybrids: from basics towards sensors. Phys Chem Chem Phys 14:9282–9290
West JL, Halas NJ (2003) Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng 5(1):285–292
Pal S, Mitra K, Azmi S, Ghosh JK, Chakraborty TK (2011) Towards the synthesis of sugar amino acid containing antimicrobial noncytotoxic cap conjugates with gold nanoparticles and a mechanistic study of cell disruption. Org Biomol Chem 9:4806–4810
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. ChemInform 36(27):1025–1102
Katz E, Willner I (2004) Integrated nanoparticle biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem Int Ed 43(45):6042–6108
Terawaki A, Otsuka Y, Lee H, Matsumoto T, Tanaka H, Kawai T (2005) Conductance measurement of a DNA network in nanoscale by point contact current imaging atomic force microscopy. Appl Phys Lett 86(11):113,901–113,903
Rodriguez-Vazquez MJ, Blanco MC, Lourido R, Vazquez-Vazquez C, Pastor E, Planes GA, Rivas J, Lopez-Quintela MA (2008) Synthesis of atomic gold clusters with strong electrocatalytic activities. Langmuir 24(21):12,690–12,694
Antoine R, Bertorelle F, Broyer M, Compagnon I, Dugourd P, Kulesza A, Mitrić R, Bonačić Koutecký V (2009) Gas-phase synthesis and intense visible absorption of tryptophan†gold cations. Angew Chem Int Ed 48(42):7829–7832
Lynch I, Dawson KA (2008) Protein-nanoparticle interactions. Nano Today 3(12):40–47
Hayat MA (ed) (1989) Colloidal gold: principles, methods, and applications, 1st edn. Academic, New York
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112(5):2739–2779
Krishnan N, Dickman MB, Becker DF (2008) Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 44(4):671–681
Lang F (2007) Mechanisms and significance of cell volume regulation. J Am Coll Nutr 26(suppl 5):613S–623S
Zhao Y, Truhlar DG (2005) How well can new-generation density functional methods describe stacking interactions in biological systems? Phys Chem Chem Phys 7:2701–2705
Zhao Y, Truhlar DG (2007) Density functionals for noncovalent interaction energies of biological importance. J Chem Theor Comput 3(1):289–300
Knal A, Acar N (2010) A DFT and TD-DFT study on intermolecular charge transfer complexes of pyrene with phenothiazine and promazine. J Mol Str THEOCHEM 949(13):36–40
Fernández EM, Soler JM, Garzón IL, Balbás LC (2004) Trends in the structure and bonding of noble metal clusters. Phys Rev B 70:165,403
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91(14):146401
Ehlers A, Bhme M, Dapprich S, Gobbi A, Hllwarth A, Jonas V, Khler K, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals ScCu, YAg and LaAu. Chem Phys Lett 208(12):111–114
Dolg M, Wedig U, Stoll H, Preuss H (1987) Energy-adjusted ab initio pseudopotentials for the first row transition elements. J Chem Phys 86(2):866–872
Andrae D, Huermann U, Dolg M, Stoll H, Preu H (1990) Energy-adjusted ab-initio pseudopotentials for the second and third row transition elements. Theor Chim Acta 77:123–141
Fernández EM, Soler JM, Balbás LC (2006) Planar and cagelike structures of gold clusters: density-functional pseudopotential calculations. Phys Rev B 73:235,433
Hariharan P, Pople J (1974) Accuracy of AH n equilibrium geometries by single determinant molecular orbital theory. Mol Phys 27(1):209–214
Hariharan P, Pople J (1972) The effect of d-functions on molecular orbital energies for hydrocarbons. Chem Phys Lett 16(2):217–219
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA (2001) 6–31 G* basis set for third-row atoms. J Comput Chem 22(9):976–984
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. xii. further extensions of gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56(5):2257–2261
Dennington R, Keith T, Millam J (2009) GaussView Version 5. Semichem Inc. Shawnee Mission, KS
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2004) NBO Version 3.1. Gaussian, Inc., Wallingford, CT
Weinhold F, Landis CR (2001) Natural bond orbitals and extensions of localized bonding concepts. Chem Educ Res Pract 2:91–104
Todd A Keith TG (2012) AIMAll (version 12.09.23). Overland Park, KS
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision C.01. Gaussian Inc, Wallingford, CT
Gimeno MC (2009) The chemistry of gold. Wiley-VCH,Weinheim, pp 1–63
Pakiari AH, Jamshidi Z (2007) Interaction of amino acids with gold and silver clusters. J Phys Chem A 111(20):4391–4396
Joshi P, Shewale V, Pandey R, Shanker V, Hussain S, Karna SP (2011) Tryptophan gold nanoparticle interaction: a first-principles quantum mechanical study. J Phys Chem C 115(46):22,818–22,826
Xie HJ, Lei QF, Fang WJ (2012) Intermolecular interactions between gold clusters and selected amino acids cysteine and glycine: a DFT study. J Mol Model 18:645–652
Pyykkö P (2008) Theoretical chemistry of gold. iii. Chem Soc Rev 37:1967–1997
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Comput Mol Sci 2(1):1–42
Bader RFW (1990) Atoms in molecules, 1st edn. Oxford University Press, Oxford
Bader R, Keith T, Gough K, Laidig K (1992) Properties of atoms in molecules: additivity and transferability of group polarizabilities. Mol Phys 75(5):1167–1189
Richardson NA, Wesolowski SS, Schaefer HF (2002) Electron affinity of the guaninecytosine base pair and structural perturbations upon anion formation. J Am Chem Soc 124(34):10,163–10,170
Crespo-Hernndez CE, Close DM, Gorb L, Leszczynski J (2007) Determination of redox potentials for the WatsonCrick base pairs, DNA nucleosides, and relevant nucleoside analogues. J Phys Chem B 111(19):5386–5395
Shukla MK, Dubey M, Zakar E, Leszczynski J (2009) DFT investigation of the interaction of gold nanoclusters with nucleic acid base guanine and the watson crick guanine-cytosine base pair. J Phys Chem C 113(10):3960–3966
Acknowledgments
We thank the Department of Science and Technology, New Delhi, Government of India, for financial support. One of the authors (Sandhya Rai) acknowledges (Council of Scientific and Industrial Research Junior Research Fellowship) fellowship Via 20-12/2009(ii)EU-IV.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 20.5 mb)
Rights and permissions
About this article
Cite this article
Rai, S., Singh, H. Electronic structure theory based study of proline interacting with gold nano clusters. J Mol Model 19, 4099–4109 (2013). https://doi.org/10.1007/s00894-012-1711-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1711-x