Abstract
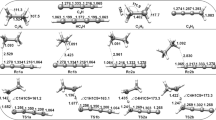
In this paper, we focus on the multiple-channel reactions of CH2XO (X = F, Cl, Br) radicals with the NO radical by means of direct dynamic methods. All structures of the stationary points were obtained at the MP2/6-311+G(d,p) level and vibrational frequency analysis was also performed at this level of theory. The minimum energy path (MEP) was obtained via the intrinsic reaction coordinate (IRC) theory at the MP2/6-311+G(d,p) level, and higher-level energetic information was refined by the MC-QCISD method. The rate constants for the three hydrogen abstraction reaction channels over the temperature range 200–1,500 K were calculated by the improved canonical variational transition state theory (ICVT) with a correction for small-curvature tunneling (SCT). The rate constants calculated in this manner were in good agreement with the available experimental data, and the three-parameter rate–temperature formulae for the temperature range 200–1,500 K were \( {k_{1{\text{a}} }}(T)=0.32\times {10^{-18 }}{T^{1.83 }}\exp \left( {1748.54/T} \right) \), \( {k_{2{\text{a}} }}(T)=0.22\times {10^{-19 }}{T^{2.19 }}\exp \left( {1770.19/T} \right) \), \( {k_{3{\text{a}} }}(T)=0.88\times {10^{-20 }}{T^{2.20 }}\exp \left( {1513.82/T} \right) \) (in units of cm3 molecule−1 s−1).





Similar content being viewed by others
References
Heeb NV, Dolezal IS, Buhrer T, Mattrel P, Wolfensberger M (1995) Chemosphere 31:3030
Tuazon EC, Atkinson R (1993) J Atmos Chem 17:179
Fuxiang W, Robert WC (2001) J Phys Chem 105:1423
McGivern WS, Kim H, Francisco JS, North SW (2004) J Phys Chem A 108:7247
Bell RL, Truong TN (1994) J Chem Phys 101:10442
Truong TN, Duncan WT, Bell RL (1996) In: Laird BB, Ross RB, Ziegler T (eds) Chemical applications of density functional theory. American Chemical Society, Washington, DC, p 85
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry: current approaches and perspectives. Kluwer, Dordrecht, p 229
Corchado JC, Espinosa-Garcia J, Hu W-P, Rossi I, Truhlar DG (1995) J Phys Chem 99:687
Hu W-P, Truhlar DG (1996) J Am Chem Soc 118:860
Boys SF, Bernardi F (1970) Mol Phys 19:553
Fast PL, Truhlar DG (2000) J Phys Chem A 104:6111
Corchado JC, Chuang Y-Y, Fast PL, Hu W-P, Liu Y-P, Lynch GC, Nguyen KA, Jackels CF, Fernandez-Ramos A, Ellingson BA, Lynch BJ, Zheng JJ, Melissasa VS, Villa J, Rossi I, Coitino EL, Pu JZ, Albu TV, Steckler R, Garrett BC, Isaacson AD, Truhlar DG (2007) Polyrate, version 9.7. Department of Chemistry and Supercomputer Institute, University of Minnesota, Minneapolis
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440
Truhlar DG, Isaacson AD, Garrett BC (1985) In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC, Boca Raton, p 65
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:275
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:281
Handy NC, Schaefer HF III (1984) J Chem Phys 81:5031
Pople JA, Raghavachari K, Schlegel HB, Binkley JS (1979) Int J Quantum Chem Quantum Chem Symp S13:225
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian, Inc., Pittsburgh
Garrett BC, Truhlar DG (1980) J Phys Chem 84:805
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Garrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235
Lovas FJ, Tiemann E (1974) J Phys Chem Ref Data 3:609
Johnson RD III (ed) Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database number 101 (August 2011 release). http://cccbdb.nist.gov/
Hammond GS (1955) J Am Chem Soc 77:334
Jacox ME, Milligan DE (1973) J Mol Spectrosc 48:536
Hisatsune IC, Heicklen J (1973) Can J Spectrosc 18:77
Shimanouchi T (2005) In: Linstrom PJ, Mallard WG (eds) NIST Chemistry WebBook, NIST Standard Reference Database number 69 (June 2005 release). http://webbook.nist.gov/chemistry/
Acknowledgments
The authors thank Professor Donald G. Truhlar for providing the program POLYRATE 9.7. This work was supported by the National Natural Science Foundation of China (20973077 and 20973049), the Program for New Century Excellent Talents in University (NCET), the Doctor Foundation by the Ministry of Education, the Doctoral Fund of Ministry of Education of China (20112303110005), the Foundation for the Department of Education of Heilongjiang Province (1152G010, 11551077), the Key Subject of Science and Technology program from the Ministry of Education of China, and the SF for Leading Experts in Academe of Harbin of China (2011RFJGS026).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, H., Chen, Q. et al. Theoretical study of the reaction of CH2XO (X = F, Cl, Br) radicals with the NO radical. J Mol Model 19, 1391–1397 (2013). https://doi.org/10.1007/s00894-012-1699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1699-2