Abstract
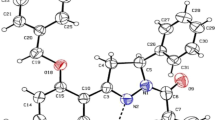
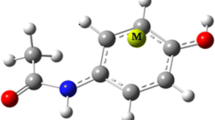
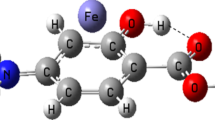
In this work several quantum properties including the NEDA and QTAIM are computed on three models of rapta-C complexes using DFT with hybrid functional and basis set with ECP and without ECP. Several interesting correlations within the observed properties and also with the reported experimental behaviors of these complexes including their biological activities are presented. The study shows that the stability of the two complexes with bidentate ligands is associated with their high hydrogen bonding stability and existence of stronger non-covalent metal-ligand bonds. The energy decomposition analysis indicated that inter-atomic interactions in the three forms of rapta-C complexes and their stability are governed by the charge transfer term with significant contributions from polarization and electrostatic terms. The higher stability of complex 1 and 2 over 3 comes from the lower exchange repulsion and higher polarization contributions to their stability which agrees perfectly with the experimental observation. Our results provide insight into the nature of intramolecular forces that influence the structural stability of the three complexes.



Similar content being viewed by others
References
Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, Zorbas H, Dyson PJ, Keppler BK (2008) KP1019, a new redox-active anticancer agent—preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers 5:2140–2155
Chatterjee S, Kundu S, Bhattacharyya A, Hartinger CG, Dyson PJ (2008) The ruthenium(II)–arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53–JNK pathways. J Biol Inorg Chem 13:1149–1155
Allardyce CS, Dorcier A, Scolaro C, Dyson PJ (2005) Development of organometallic (organo-transition metal) pharmaceuticals. Appl Organometal Chem 19:1–10
Gasser G, Ott I, Metzler-Nolte N (2011) Organometallic anticancer compounds. J Med Chem 54:3–25
Ang WH, Daldini E, Scolaro C, Scopelliti R, Juillerat-Jeannerat L, Dyson PJ (2006) Development of organometallic ruthenium−Arene anticancer drugs that resist hydrolysis. Inorg Chem 45:9006–9013
Pratuangdejkul J, Pascale Jaudon P, Ducrocq OC (2006) Cation-π interactions in serotonin: conformational, electronic distribution, and energy decomposition analysis. J Chem Theory Comput 2:746–760
Ciancetta A, Genheden S, Ryde U (2011) A QM/MM study of the binding of RAPTA ligands to cathepsin B. J Comput Aided Mol Des 25:729–742
Gossens C, Dorcier A, Dyson PJ, Rothlisberger U (2007) pKa estimation of ruthenium(II)-Arene PTA complexes and their hydrolysis products via a DFT/continuum electrostatics approach. Organometallics 26:3969–3975
Deubel DV, Lau JK (2006) In silico evolution of substrate selectivity: comparison of organometallic ruthenium complexes with the anticancer drug cisplatin. Chem Commun 23:2451–2453
Alkorta I, Blanco F, Elguero J, Dobado JA, Ferrer SM, Vidal I (2009) Carbon · · · Carbon weak interactions. J Phys Chem A 113:8387–8393
Keith TA (2012) AIMAll (Version 12.06.03), TK Gristmill Software, Overland Park KS, USA, (aim.tkgristmill.com)
Platts JA, Overgaard J, Jones C, Iversen BB, Stasch A (2011) First experimental characterization of a non-nuclear attractor in a dimeric magnesium(I) compound. J Phys Chem A 115:194–200
Timerghazin QK, Rizvi I, Peslherbe GH (2011) Can a dipole-bound electron form a pseudo-atom? An atoms-in-molecules study of the hydrated electron. J Phys Chem A 115:13201–13209
Granovsky AA Firefly version 7.1.G, www http://classic.chem.msu.su/gran/firefly/index.html
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian, Inc., Pittsburgh PA
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) Gamess (US). J Comput Chem 14:1347–1363
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 Model. J Chem Phys 110:6158–6170
Feller DJ (1996) The role of databases in support of computational chemistry calculations. Comp Chem 17(13):1571–1586
Schuchardt KL, Didier BT, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL (2007) Basis set exchange: a community database for computational sciences. J Chem Inf Model 47(3):1045–1052
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Relativistic compact effective potentials and efficient, shared-exponent basis sets for the third-, fourth-, and fifth-row atoms. Can J Chem 70:612–630
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Dobbs KD, Hehre WJ (1987) Molecular orbital theory of the properties of inorganic and organometallic compounds 5. Extended basis sets for first-row transition metals. J Comp Chem 6:861–879
Marchal B, Carbonniére P, Begue D, Pouchan C (2008) Structural and vibrational determination of small gallium–arsenide clusters from CCSD(T) and DFT calculations. Chem Phy Lett 453:49–54
Marchal R, Carbonnière P, Pouchan C (2012) Structural and vibrational properties prediction of SnnTen clusters (n = 2–8) using the GSAM approach. Comput Theoret Chem 990:100–105, In press
Bagno A, Bonchio M (2002) DFT calculations of 99Ru chemical shifts with all-electron and effective core potential basis sets. Eur J Inorg Chem. 1475–1483
Foster JP, Weinhold FJ (1980) Natural hybrid orbitals. Am Chem Soc 102:7211–7218
Glendening ED, Streitwieser AJ (1994) Natural energy decomposition analysis: an energy partitioning procedure for molecular interactions with application to weak hydrogen bonding, strong ionic, and moderate donor–acceptor interactions. Chem Phys 100:2900–2909
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–745
Sebastian S, Sundaraganesan N (2010) The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim Acta Part A 75(3):941–952
Calhorda MJ, Lopes PEM (2000) An ‘atoms in molecules’ (AIM) analysis of the dihydrogen bond in organometallic compounds. J Organometal Chem 609:53–59
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Edgecombe KE, Esquivel RO, Smith VH Jr, Muller-Plathe F (2002) Pseudoatoms of the electron density. J Chem Phys 97:2593–2599
de Silva IC, de Silva RM, de Silva KMN (2005) Investigations of nonlinear optical (NLO) properties of Fe, Ru and Os organometallic complexes using high accuracy density functional theory (DFT) calculations. J Mol Struct Theochem 728:141–145
Mendesa PJ, Ramalho JPP, Candeias AJE, Robalod MP, Garcia MH (2005) Density functional theory calculations on h5-monocyclopentadienylnitrilecobalt complexes concerning their second-order nonlinear optical properties. J Mol Struct Theochem 729:109–113
Liyanage PS, de Silva RM, de Silva KMN (2003) Nonlinear optical (NLO) properties of novel organometallic complexes: high accuracy density functional theory (DFT) calculations. J MolStruc (Theochem) 639:195–201
Zeng T, Klobukowski M (2008) Relativistic model core potential study of the Au+Xe system. J Phys Chem A 112:5236–5242
Labello NP, Ferreira AM, Kurtz HA (2006) Utilizing relativistic effective core potentials for accurate calculations of molecular polarizabilities on transition metal compounds. J Phys Chem A 110:13507–13513
Gaur R, Mishra L (2012) Synthesis and characterization of Ru(II)−DMSO−Cl−chalcone complexes: DNA binding, nuclease, and topoisomerase II inhibitory activity. Inorg Chem 51:3059–3070
Stepanenko IN, Casini A, Edafe F, Novak MS, Arion VB, Dyson PJ, Jakupec MA, Keppler BK (2011) Conjugation of Organoruthenium(II) 3-(1H-Benzimidazol-2-yl)pyrazolo[3,4-b]pyridines and Indolo[3,2-d]benzazepines to recombinant human serum albumin: a strategy to enhance cytotoxicity in cancer cells. Inorg Chem 50:12669–12679
Tiana D (2009/2010) Organometallic chemistry from the interacting quantum atoms approach, Ph.D. Thesis, Università degli Studi di Milano, Milano, pp 18–19, 46
Farrugia LJ, Senn HM (2012) On the unusual weak intramolecular C…C interactions in Ru3(CO)12: a case of bond path artifacts introduced by the Multipole model? J Phys Chem A 116:738–746
Acknowledgments
The authors gratefully acknowledged the financial support of Govan Mbeki Research and Development Centre, University of Fort Hare, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adeniyi, A.A., Ajibade, P.A. Effects of bidentate coordination on the molecular properties rapta-C based complex using theoretical approach. J Mol Model 19, 1325–1338 (2013). https://doi.org/10.1007/s00894-012-1683-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1683-x