Abstract
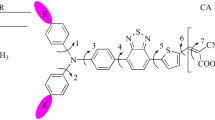
The ground state geometries have been computed by using density functional theory. The excitation energies for dye sensitizers were performed by using time dependant density functional theory. The polarizable continuum model (PCM) has been used for evaluating bulk solvent effects at all stages. The calculations have been carried out in methanol according to the experimental set up. The long-range-corrected functional (PCM-TD-LC-BLYP) underestimate the absorption spectrum of parent molecule while PCM-TDBHandHLYP is in good agreement with the experimental data. The highest occupied molecular orbital (HOMO) is delocalized on TPA moiety while lowest unoccupied molecular orbital (LUMO) is localized on anchoring group, conjugated chain and the benzene ring near to the anchoring group. The LUMO energies of all the investigated dyes are above the conduction band of TiO2, HOMOs are below the redox couple and HOMO-LUMO energy gaps of studied dyes are smaller compared to TC4. The 1 and 3 are 7 and 12 nm blue shifted while 2 and 4 are 25 and 22 nm red shifted, respectively compared to TC4. The trend of electron injection (ΔGinject), relative electron injection (ΔG injectr ), and electronic coupling constant (|VRP|) has been observed as 3 > 1 > 4 > 2 > TC4. The improved ΔGinject, |VRP| and light harvesting efficiency (LHE) of new designed sensitizers revealed that these materials would be excellent sensitizers. The broken coplanarity between the benzene near anchoring group having LUMO and the last benzene attached to TPA unit in 1–4 consequently would hamper the recombination reaction.

HOMOs are delocalized on donor moieties while LUMOs are localized towards anchoring groups. The comprehensible charge transfer has been observed from donor to acceptor side. The elongation of the bridge leads to higher the HOMO energies, lower the LUMO energies and decrease the energy gap. The LUMO energies are above the conduction band of TiO2, and HOMO energies below the redox couple. Improved electron injection, electronic coupling constant and light harvesting efficiency revealed that designed materials would be efficient.



Similar content being viewed by others
References
Regan BO, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye- sensitized colloidal TiO2 films. Nature 353:737–740
Nazeeruddin MK, De Angelis F, Fantacci S, Selloni A, Viscardi G, Liska P, Ito S, Takeru B, Grätzel M (2005) Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J Am Chem Soc 127:16835–16847
Zhang QF, Dandeneau CS, Zhou XY, Cao GZ (2009) ZnO nanostructures for dye-sensitized solar cells. Adv Mater 21:4087–4108
Li G, Jiang KJ, Li YF, Li SL, Yang LM (2008) Efficient structural modification of triphenylamine-based organic dyes for dye-sensitized solar cells. J Phys Chem C 112:11591–11599
Wong BM, Codaro JG (2008) Coumarin dyes for dye-sensitized solar cells: a long-range-corrected density functional study. J Chem Phys 129:214703–214710
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) High efficiency of dye-sensitized solar cells based on metal-free indoline dyes. J Am Chem Soc 126:12218–12219
Stathatos E, Lianos P, Laschewsky A, Ouari O, Van Cleuvenbergen P (2001) Synthesis of a hemicyanine dye bearing two carboxylic groups and its use as a photosensitizer in dye-Sensitized photoelectrochemical cells. Chem Mater 13:3888–3892
Baik C, Kim D, Kang MS, Song K, Sang OK, Ko J (2009) Synthesis and photovoltaic properties of novel organic sensitizers containing indolo[1,2-f]phenanthridine for solar cell. Tetrahedron 65:5302–5307
Ferrere S, Zaban A, Gregg B (1997) Dye sensitization of nanocrystalline tin oxide by perylene derivatives. J Phys Chem B 101:4490–4493
Nilsing M, Persson P, Lunell S, Ojamäe L (2007) Dye-sensitization of the TiO2 rutile (110) surface by perylene dyes: Quantum-chemical periodic B3LYP computations. J Phys Chem C 111:12116
Robertson N (2006) Optimizing dyes for dye-sensitized solar cells. Angew Chem Int Ed 45:2338–2345
Liu D, Fessenden RW, Hug GL, Kamat PV (1997) Dye capped semiconductor nanoclusters. Role of back electron transfer in the photosensitization of SnO2 nanocrystallites with cresyl violet aggregates. J Phys Chem B 101:2583–2590
Burfeindt B, Hannappel T, Storck W, Willig F (1996) Measurement of temperature-independent femtosecond interfacial electron transfer from an anchored molecular electron donor to a semiconductor as acceptor. J Phys Chem 100:16463–16465
Sayama K, Tsukagochi S, Hara K, Ohga Y, Shinpou A, Abe Y, Suga S, Arakawa H (2002) Photoelectrochemical properties of J aggregates of benzothiazole merocyanine dyes on a nanostructured TiO2 film. J Phys Chem B 106:1363–1371
Hagfeldt A, Gratzel M (2000) Molecular photovoltaics. Acc Chem Res 33:269–277
Ning Z, Zhang Q, Wu W, Pei H, Liu B, Tian H (2008) Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J Org Chem 73:3791–3797
Xu W, Peng B, Chen J, Liang M, Cai F (2008) New triphenylamine-based dyes for dye-sensitized solar cells. J Phys Chem C 112:874–880
Irfan A, Al-Sehemi AG, Asiri AM (2012) Donor-enhanced bridge effect on the electronic properties of triphenylamine based dyes: density functional theory investigations. J Mol Model. doi:10.1007/s00894-012-1372-9
Irfan A, Cui R, Zhang J, Hao L (2009) Push–pull effect on the charge transfer, and tuning of emitting color for disubstituted derivatives of mer-Alq3. Chem Phys 364:39–45
Preat J, Michaux C, Jacquemin D, Perpete EA (2009) Enhanced efficiency of organic dye-sensitized solar cells: triphenylamine derivatives. J Phys Chem C 113:16821–16833
Irfan A, Aftab H, Al-Sehemi AG (2012) Push–pull effect on the geometries, electronic and optical properties of thiophene based dye-sensitized solar cell materials. J Saudi Chem Soc. doi:10.1016/j.jscs.2011.11.013
Al-Sehemi AG, Irfan A, Asiri AM, Ammar YA (2012) Synthesis, characterization and DFT study of methoxybenzylidene containing chromophores for DSSC materials. Spectrochim Acta A 91:239–243
Frisch MJ et al (2009) Gaussian 09, Revision A.1. Gaussian Inc, Wallingford, CT
Becke AD (1993) Density–functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157:200–206
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Sun J, Song J, Zhao Y, Liang WZ (2007) Real-time propagation of the reduced one-electron density matrix in atom-centered Gaussian orbitals: application to absorption spectra of silicon clusters. J Chem Phys 127:234107–234113
Zhang CR, Liang WZ, Chen HS, Chen YH, Wei ZQ, Wu YZ (2008) Theoretical studies on the geometrical and electronic structures of N-methyle-3,4-fulleropyrrolidine. J Mol Struct THEOCHEM 862:98–104
Matthews D, Infelta P, Grätzel M (1996) Sol Energy Mater Sol Cells 44:119–155
Cossi M, Barone V (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115:4708–4717
Amovilli C, Barone V, Cammi R, Cancès E, Cossi M, Mennucci B, Pomelli CS, Tomasi J (1998) Recent advances in the description of solvent effects with the polarizable continuum model. Adv Quant Chem 32:227–261
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Peach MJG, Benfield P, Helgaker T, Tozer DJ (2008) Excitation energies in density functional theory: an evaluation and a diagnostic test. J Chem Phys 128:044118
Stein T, Kronik L, Baer R (2009) Prediction of charge-transfer excitations in coumarin-based dyes using a range-separated functional tuned from first principles. J Chem Phys 131:244119–244123
Wong BM, Piacenza M, Sala FD (2009) Absorption and fluorescence properties of oligothiophene biomarkers from long-range-corrected time-dependent density functional theory. Phys Chem Chem Phys 11:4498–4508
Wong BM, Cordaro JG (2008) Coumarin dyes for dye-sensitized solar cells: a long-range-corrected density functional study. J Chem Phys 129:214703–214710
Preat J, Jacquemin D, Perpete E (2010) Design of new triphenylamine-sensitized solar cells: a theoretical approach. Environ Sci Technol 44:5666–5671
Balanay MP, Kim DH (2008) DFT/TD-DFT molecular design of porphyrin analogues for use in dye-sensitized solar cells. Phys Chem Chem Phys 10:5121–5127
De Angelis F, Fantacci S, Selloni A (2008) Alignment of the dye's molecular levels with the TiO2 band edges in dye-sensitized solar cells: a DFT–TDDFT study. Nanotechnology 19:424002–424008
Ito S, Zakeeruddin SM, Humphry-Baker R, Liska P, Charvet R, Comte P, MdK N, Pechy P, Takata M, Miura H, Uchida S, Gratzel M (2006) High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv Mater 18:1202–1205
Matthews D, Infelta P, Grätzel M (1996) Calculation of the photocurrent-potential characteristic for regenerative, sensitized semiconductor. Sol Energ Mater Sol Cells 44:119–155
Pourtois G, Beljonne J, Ratner MA, Bredas JL (2002) Photoinduced electron-transfer processes along molecular wires based on phenylenevinylene oligomers: a quantum-chemical insight. J Am Chem Soc 124:4436–4447
Hsu C (2009) The electronic couplings in electron transfer and excitation energy transfer. Acc Chem Res 42:509–518
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65:599–610
Higendorff M, Sundstrom V (1998) Dynamics of electron injection and recombination of dye-sensitized TiO2 particles. J Phys Chem B 102:10505–10514
Asbury JB, Wang YQ, Hao E, Ghosh H, Lian T (2001) Evidences of hot excited state electron injection from sensitizer molecules to TiO2 nanocrystalline thin films. Res Chem Intermed 27:393–406
Katoh R, Furube A, Yoshihara T, Hara K, Fujihashi G, Takano S, Murata S, Arakawa H, Tachiya M (2004) Efficiencies of electron injection from excited N3 dye into nanocrystalline semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) films. J Phys Chem B 108:4818–4822
Hagfeldt A, Grätzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Barbara PF, Meyer TJ, Ratner MA (1996) Contemporary issues in electron transfer research. J Phys Chem 100:13148–13168
De Angelis F, Fantacci S, Selloni A (2008) Alignment of the dye's molecular levels with the TiO2 band edges in dye-sensitized solar cells: a DFT–TDDFT study. Nanotechnology 19:424002–424008
Benkö G, Kallioien J, Korppi-Tommola JEI, Yartsev AP, Sundström V (2002) Photoinduced ultrafast dye-to-semiconductor electron injection from nonthermalized and thermalized donor states. J Am Chem Soc 124:489–493
Iwa S, Hara K, Murata S, Katoh R, Sugihara H, Arakawa H (2000) Ultrafast interfacial charge separation processes from the singlet and triplet MLCT states of Ru(bpy)2(dcbpy) adsorbed on nanocrystalline SnO2 under negative applied bias. J Chem Phys 113:3366–3373
Preat J, Michaux C, Jacquemin D, Perpète EA (2009) Enhanced efficiency of organic dye-sensitized solar cells: triphenylamine derivatives. J Phys Chem C 113:16821–16833
Nalwa HS (2001) Handbook of advanced electronic and photonic materials and devices. Academic, San Diego
Cassida M (1995) Recent advances in density functional methods: time dependent density functional response theory for molecules. Chong, Singapore
Harris DC, Bertolucci MD (1998) Symmetry and spectroscopy. Dover, New York
Acknowledgements
We are thankful to the King Khalid University for the support and facilities provided to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Irfan, A., Al-Sehemi, A.G. Quantum chemical study in the direction to design efficient donor-bridge-acceptor triphenylamine sensitizers with improved electron injection. J Mol Model 18, 4893–4900 (2012). https://doi.org/10.1007/s00894-012-1488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1488-y