Abstract
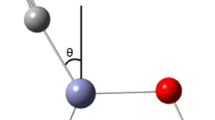
A theoretical investigation of the adsorption of CO2 onto ZrO2 is presented. Various cluster models were used to mimic different basic and acidic sites on the surface. The method used was the density functional theory with the generalized gradient approximation and including Grimme’s empirical model in order to properly describe the weak interactions that may occur between the adsorbate and the surface. We found that the adsorption at sites exhibiting two adjacent unsaturated zirconium atoms led to either the exothermic dissociation of CO2 or to a strongly physisorbed state. By contrast, on a single unsaturated zirconium, CO2 was adsorbed in an apical manner. In this case, the molecule is highly polarized and the adsorption energy amounts to −64.6 kJ mol−1. Finally, the weakest adsorption of CO2 occurred on the basic OH sites on the surface.








Similar content being viewed by others
References
Tanabe K (1985) Surface and catalytic properties of ZrO2. Mater Chem Phys 13:347–364
Sohn YH, Biederman RR, Sisson RD Jr (1994) Microstructural development in physical vapour-deposited partially stabilized zirconia thermal barrier coatings. Thin Solid Films 250:1–7
He MY, Ekerdt JC (1984) Methanol formation on zirconium dioxide. J Catal 90:17–23
Yamaguchi T, Hightower JW (1977) Hydrogenation of 1,3-butadiene with 1,3-cyclohexadiene and molecular deuterium over zirconium dioxide catalysts. J Am Chem Soc 99:4201–4203
Nakano Y, Iizuka T, Hattori H, Tanabe K (1979) Surface properties of zirconium oxide and its catalytic activity for isomerization of 1-butene. J Catal 57:1–10
Davis BH, Ganesan P (1979) Catalytic conversion of alcohols. 11. Influence of preparation and pretreatment on the selectivity of zirconia. Ind Eng Chem Prod Res Dev 18:191–198
Wan KT, Khouw CB, Davis ME (1996) Studies on the catalytic activity of zirconia promoted with sulfate, iron, and manganese. J Catal 158:311–326
Audry F, Hoggan PE, Saussey J, Lavalley JC, Lauron-Pernot H, Le Govic AM (1997) Infrared study and quantum calculations of the conversion of methylbutynol into hydroxymethylbutanone on zirconia. J Catal 168:471–481
Trovarelli A, Zamar F, Llorca J, de Leitenburg C, Dolcetti G, Kiss JT (1997) Nanophase fluorite-structured CeO2-ZrO2 catalysts prepared by high-energy mechanical milling. J Catal 169:490–502
Stichert W, Schuth F (1998) Synthesis of catalytically active high surface area monoclinic sulfated zirconia. J Catal 174:242–245
Tret’yakov NE, Pozdnyakov DV, Oranskaya OM, Filimonov VN (1970) Adsorption of some molecules on zirconium dioxide studied by an infrared spectroscopic method. Russ J Phys Chem 44:596–600
He MY, Ekerdt JG (1984) Infrared studies of the adsorption of synthesis gas on zirconium dioxide. J Catal 87:381–388
Kondo J, Abe H, Sakata Y, Maruya K, Domen K, Onishi T (1988) Infrared studies of adsorbed species of H2, CO and CO2 over ZrO2. J Chem Soc Faraday Trans 84:511–519
Hertl W (1989) Surface chemistry of zirconia polymorphs. Langmuir 5:96–100
Morterra C, Orio L (1990) Surface characterization of zirconium oxide. II. The interaction with carbon dioxide at ambient temperature. Mater Chem Phys 24:247–268
Cerrato G, Bordiga S, Barbera S, Morterra C (1997) A surface study of monoclinic zirconia (m-ZrO2). Surf Sci 377:50–55
Aboulayt A, Maugé F, Hoggan PE, Lavalley JC (1996) Combined FTIR, reactivity and quantum chemistry investigation of COS hydrolysis at metal oxide surfaces used to compare hydroxyl group basicity. Catal Lett 39:213–218
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401
Perdew JP, Kurth S, Zupan A, Blaha P (1999) Accurate density functional with correct formal properties: a step beyond the generalized gradient approximation. Phys Rev Lett 82:2544–2547
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2003) Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes. J Chem Phys 119:12129–12137
Zhao Y, Truhlar DG (2005) Benchmark databases for nonbonded interactions and their use to test density functional theory. J Chem Theor Comput 1:415–432
Kanai Y, Wang X, Selloni A, Car R (2006) Testing the TPSS meta-generalized-gradient-approximation exchange-correlation functional in calculations of transition states and reaction barriers. J Chem Phys 125:234104
Zhao Y, Truhlar DG (2006) Comparative DFT study of van der Waals complexes: rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers. J Phys Chem A 110:5121–5129
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Schaefer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian-basis sets for atoms Li to Kr. J Chem Phys 97:2571–2578
Boys SF, Bernardi F (1970) Calculation of small molecular interactions by differences of separate total energies—some procedures with reduced errors. Mol Phys 19:553–566
Whitten JL (1973) Coulombic potential energy integrals and approximations. J Chem Phys 58:4496–4502
Baerends EJ, Ellis DE, Ros P (1973) Self-consistent molecular Hartree–Fock–Slater calculations. I. The computational procedure. Chem Phys 2:41–51
Dunlap BI, Connolly JWD, Sabin JR (1979) On some approximations in applications of Xalpha theory. J Chem Phys 71:3396–3403
Van Alsenoy C (1988) Ab initio calculations on large molecules: the multiplicative integral approximation. J Comput Chem 9:620–626
Eichkorn K, Treutler O, Öhm H, Häser M, Ahlrichs R (1995) Auxiliary basis sets to approximate Coulomb potentials. Chem Phys Lett 240:283–289
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor Chem Acc 97:119–124
Kendall RA, Früchtl HA (1997) The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor Chem Acc 97:158–163
Neese F (2011) The ORCA program system. Comput Mol Sci 00:1–6
Teufer G (1962) The crystal structure of tetragonal ZrO2. Acta Crystallogr 15:1187
Patil RN, Subbarao EC (1970) Monoclinic-tetragonal phase transition in zirconia: mechanism, pretransformation and coexistence. Acta Crystallogr A 26:535–542
Jing-Lin Y, Guo-Chang J, Song-Hua Y, Jin-Chang M, Kuang-Di X (2001) Temperature dependence of the Raman spectra and phase transition of zirconia. Chin Phys Lett 18:991–993
Sekulić A, Furić K, Stubičar M (1997) Raman study of phase transitions in pure and alloyed zirconia induced by ball-milling and a laser beam. J Mol Struct 410–411:275–279
Gripp J, Mader H, Dreizler H, Teffo JL (1995) The microwave spectrum of carbon dioxide 17OCO and 18OCO. J Mol Spectrosc 172:430–434
Fuentealba P, Simón-Manso Y (1999) Basis set superposition error in atomic cluster calculations. Chem Phys Lett 314:108–113
Gilliam OR, Johnson CM, Gordy W (1950) Microwave spectroscopy in the region from two to three millimeters. Phys Rev 78:140–144
Freund HJ, Roberts MW (1996) Surface chemistry of carbon dioxide. Surf Sci Rep 25:225–273
Bachiller-Baeza B, Rodriguez-Ramos I, Guerrero-Ruiz A (1998) Interaction of carbon dioxide with the surface of zirconia polymorphs. Langmuir 14:3556–3564
Wang SG, Liao XY, Cao DB, Huo CF, Li YW, Wang J, Jiao H (2007) Factors controlling the interaction of CO2 with transition metal surfaces. J Phys Chem C 111:16934–16940
Markovits A, Fahmi A, Minot C (1996) A theoretical study of CO2 adsorption on TiO2. J Mol Struct (THEOCHEM) 371:219–235
Ahdjoudj J, Markovits A, Minot C (1999) Hartree–Fock periodic study of the chemisorption of small molecules on TiO2 and MgO surfaces. Catal Today 50:541–551
Takahashi H, Yuki K, Nitta T (2002) Chemical modification of rutile TiO2(110) surface by ab initio calculations for the purpose of CO2 adsorption. Fluid Phase Equilibr 194–197:153–160
Melle-Franco M, Pacchioni G, Chadwick AV (2001) Cluster and periodic ab initio calculations on the adsorption of CO2 on the SnO2(110) surface. Surf Sci 478:25–34
Lopes Martins JB, Longo E, Rodríguez Salmon OD, Espinoza VAA, Taft CA (2004) The interaction of H2, CO, CO2, H2O and NH3 on ZnO surfaces: an ONIOM study. Chem Phys Lett 400:481–486
Kotsis K, Stodt D, Staemmler V, Kovacik R, Meyer B, Traeger F, Langenberg D, Strunskus T, Kunat M, Wöll C (2008) CO2 adlayers on the mixed terminated ZnO(10-10) surface studied by he atom scattering, photoelectron spectroscopy and ab initio electronic structure calculations. Z Phys Chem 222:891–915
Schneider WF (2004) Qualitative differences in the adsorption chemistry of acidic (CO2, SOx) and amphiphilic (NOx) species on the alkaline earth oxides. J Phys Chem B 108:273–282
Acknowledgments
The author P. Boulet is grateful to the Centre Informatique National de l’Enseignement Supérieur (CINES, Montpellier, France) and the Centre Régional de Compétences en Modélisation Moléculaire (CRCMM, Marseille, France) for providing access to their computer facilities. This work was granted access to the HPC resources of CINES under the allocation 2011085120 made by GENCI (Grand Equipement National de Calcul Intensif).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boulet, P., Knöfel, C., Kuchta, B. et al. Computational investigation of the adsorption of carbon dioxide onto zirconium oxide clusters. J Mol Model 18, 4819–4830 (2012). https://doi.org/10.1007/s00894-012-1486-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1486-0