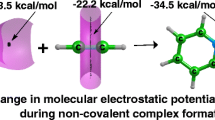
Abstract
MP2(full)/6-311++G(3df,3pd) calculations were carried out on complexes linked through various non-covalent Lewis acid – Lewis base interactions. These are: hydrogen bond, dihydrogen bond, hydride bond and halogen bond. The quantum theory of ´atoms in molecules´ (QTAIM) as well as the natural bond orbitals (NBO) method were applied to analyze properties of these interactions. It was found that for the A-H…B hydrogen bond as well as for the A-X…B halogen bond (X designates halogen) the complex formation leads to the increase of s-character in the A-atom hybrid orbital aimed toward the H or X atom. In opposite, for the A…H-B hydride bond, where the H-atom possesses negative charge, the decrease of s-character in the B-atom orbital is observed. All these changes connected with the redistribution of the electron charge being the effect of the complex formation are in line with Bent´s rule. The numerous correlations between energetic, geometrical, NBO and QTAIM parameters were also found.

QTAIM atomic radii for NH4 +…HMgH and Na+…HBeH






Similar content being viewed by others
References
Murray JS, Riley KE, Politzer P, Clark T (2010) Aust J Chem 63:1598–1607
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, Berlin
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York
Grabowski SJ (ed) (2006) Hydrogen bonding – new insights. Vol.3 of the series: challenges and advances in computational chemistry and physics. In: Leszczynski J (ed) Springer, Dordrecht
Lipkowski P, Grabowski SJ, Leszczynski J (2006) J Phys Chem A 110:10296–10302
Metrangolo P, Resnati G (2001) Chem Eur J 7:2511–2519
Formigué M, Batail P (2004) Chem Rev 104:5379–5418
Zordan F, Brammer L, Sherwood P (2005) J Am Chem Soc 127:5979–5989
Clark T, Hennemann M, Murray J, Politzer P (2007) J Mol Model 13:291–296
Peris E, Lee JC Jr, Rambo J, Eisenstein O, Crabtree RH (1995) J Am Chem Soc 117:3485–3491
Wessel J, Lee JC Jr, Peris E, Yap GPA, Fortin JB, Ricci JS, Sini G, Albinati A, Koetzle TF, Eisenstein O, Rheingold AL, Crabtree RH (1995) Angew Chem Int Ed Engl 34:2507–2509
Crabtree RH, Siegbahn PEM, Eisenstein O, Rheingold AL, Koetzle TF (1996) Acc Chem Res 29:348–354
Bakhmutow VI (2008) Dihydrogen bonds. Wiley, New Jersey
Alkorta I, Rozas I, Elguero J (1998) Chem Soc Rev 27:163–170
Rozas I, Alkorta I, Elguero J (1997) J Phys Chem A 101:4236–4244
Cotton FA, Matonic JH, Murillo CA (1998) J Am Chem Soc 120:6047–6052
Grabowski SJ, Sokalski WA, Leszczynski J (2006) Chem Phys Lett 422:334–339
Scheiner S (2011) J Chem Phys 134:094315–094323
Scheiner S (2011) J Phys Chem A 115:11202–11209
Weinhold F, Landis C (2005) Valency and bonding, a natural bond orbital donor – acceptor perspective. Cambridge University Press, Cambridge
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Weinhold F (1997) J Mol Struct THEOCHEM 398–399:181–197
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) J Mol Model 13:305–311
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033–1038
Politzer P, Murray JS, Concha MC (2008) J Mol Model 14:659–665
Murray J, Concha MC, Lane P, Hobza P, Politzer P (2008) J Mol Model 14:699–704
Murray J, Lane P, Politzer P (2009) J Mol Model 15:723–729
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748–7757
Grabowski SJ (2011) Chem Rev 11:2597–2625
Sobczyk L, Grabowski SJ, Krygowski TM (2005) Chem Rev 105:3513–3560
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) J Am Chem Soc 125:5973–5987
Grabowski SJ, Ugalde JM (2010) J Phys Chem A 114:7223–7229
Alabugin IV, Manoharan M (2006) J Comput Chem 28:373–390
Bent HA (1961) Chem Rev 61:275–311
Koch U, Popelier PLA (1995) J Phys Chem A 99:9747–9754
Popelier P (2000) Atoms in Molecules. An Introduction. Prentice Hall, Harlow UK
Grabowski SJ (2011) J Phys Chem A 115:12789–12799
Grabowski SJ (2011) J Phys Chem A 115:12340–12347
Grabowski SJ (2012) J Phys Chem A 116:1838–1845
Frisch MJ, Trucks GW et al (2009) Gaussian 09, Revision A.1. Gaussian Inc, Wallingford, CT
Gu Y, Kar T, Scheiner S (1999) J Am Chem Soc 121:9411–9422
Boys SF, Bernardi F (1979) Mol Phys 19:553–566
Grabowski SJ (2006) Annu Rep Prog Chem Sect C 102:131–165
Grabowski SJ, Sadlej AJ, Sokalski WA, Leszczynski J (2006) Chem Phys 327:151–158
Bader RFW (1985) Acc Chem Res 18:9–15
Bader RFW (1991) Chem Rev 91:893–928
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
Matta C, Boyd RJ (eds) (2007) Quantum theory of atoms in molecules: recent progress in theory and application. Wiley-VCH, Weinheim
Keith TA (2011) AIMAll (Version 11.08.23), TK Gristmill Software, Overland Park KS, USA (aim.tkgristmill.com)
Cybulski H, Pecul M, Sadlej J, Helgaker T (2003) J Chem Phys 119:5094–5104
Grabowski SJ, Sokalski WA, Dyguda E, Leszczynski J (2006) J Phys Chem B 110:6444–6446
Nishio M, Hirota M, Umezawa Y (1998) The CH/π interaction, evidence, nature, and consequences. Wiley, New York
Cremer D, Kraka E (1984) Croat Chem Acta 57:1259–1281
Rozas I, Alkorta I, Elguero J (2000) J Am Chem Soc 122:11154–11161
Joseph J, Jemmis ED (2007) J Am Chem Soc 129:4620–4632
Hobza P (2001) Phys Chem Chem Phys 3:2555–2556
Hobza P, Havlas Z (2000) Chem Rev 100:4253–4264
Acknowledgments
Financial support comes from Eusko Jaurlaritza (GIC 07/85 IT-330-07) and the Spanish Office for Scientific Research (CTQ2011-27374). Technical and human support provided by Informatikako Zerbitzu Orokora - Servicio General de Informatica de la Universidad del Pais Vasco (SGI/IZO-SGIker UPV/EHU), Ministerio de Ciencia e Innovación (MICINN), Gobierno Vasco Eusko Jaurlanitza (GV/EJ), European Social Fund (ESF) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabowski, S.J. Non-covalent interactions – QTAIM and NBO analysis. J Mol Model 19, 4713–4721 (2013). https://doi.org/10.1007/s00894-012-1463-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1463-7