Abstract
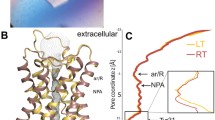
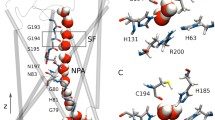
Aquaporin Z (AQPZ) is a tetrameric protein that forms water channels in the cell membrane of Escherichia coli. The histidine residue (residue 174) in the selectivity filter (SF) region plays an important role in the transport of water across the membrane. In this work, we perform equilibrium molecular dynamics (MD) simulations to illustrate the gating mechanism of the SF and the influences of residue 174 in two different protonation states: Hsd174 with the proton at Nδ, and Hse174 with the proton at Nε. We calculate the pore radii in the SF region versus the simulation time. We perform steered MD to compute the free-energy profile, i.e., the potential of mean force (PMF) of a water molecule through the SF region. We conduct a quantum mechanics calculation of the binding energy of one water molecule with the residues in the SF region. The hydrogen bonds formed between the side chain of Hsd174 and the side chain of residue 189 (Arg189) play important roles in the selectivity mechanism of AQPZ. The radii of the pores, the hydrogen-bond analysis, and the free energies show that it is easier for water molecules to permeate through the SF region of AQPZ with residue 174 in the Hse state than in the Hsd state.

Free energy profile estimated with the BD-FDT












Similar content being viewed by others
References
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Science 256:385–387
Borgnia M, Nielsen S, Engel A, Agre P (1999) Annu Rev Biochem 68:425–458
Agre P, Kozono D (2003) FEBS Lett 555:72–78
King LS, Kozono D, Agre P (2004) Nat Rev Mol Cell Biol 5:687–698
Hub JS, Grubmuller H, de Groot BL (2009) Handb Exp Pharmacol 190:57–76
Savage DF, Egea PF, Robles-Colmenares Y, O’Connell JD III, Stroud RM (2003) PLoS Biol 1:E72
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Nature 407:599–605
Sui HX, Han BG, Lee JK, Walian P, Jap BK (2001) Nature 414:872–878
Fu DX, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM (2000) Science 290:481–486
Savage DF, Stroud RM (2007) J Mol Biol 368:607–617
Savage DF, O’Connell JD III, Miercke LJ, Finer-Moore J, Stroud RM (2010) Proc Natl Acad Sci USA 107:17164–17169
Jiang JS, Daniels BV, Fu D (2006) J Biol Chem 281:454–460
Wang Y, Schulten K, Tajkhorshid E (2005) Structure 13:1107–1118
Jensen MO, Mouritsen OG (2006) Biophys J 90:2270–2284
Hashido M, Ikeguchi M, Kidera A (2005) FEBS Lett 579:5549–5552
de Groot BL, Grubmuller H (2001) Science 294:2353–2357
Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O’Connell J, Stroud RM, Schulten K (2002) Science 296:525–530
Jensen MO, Tajkhorshid E, Schulten K (2003) Biophys J 85:2884–2899
Burykin A, Warshel A (2003) Biophys J 85:3696–3706
Burykin A, Kato M, Warshel A (2003) Prot Struc Funct Gen 52:412–426
Kato M, Pisliakov AV, Warshel A (2006) Proteins 64:829–844
de Groot BL, Grubmuller H (2005) Curr Opin Struct Biol 15:176–183
Chen H, Ilan B, Wu Y, Zhu F, Schulten K, Voth GA (2007) Biophys J 92:46–60
Wang Y, Shaikh SA, Tajkhorshid E (2010) Physiology (Bethesda) 25:142–154
Calamita G (2000) Mol Microbiol 37:254–262
Borgnia MJ, Kozono D, Calamita G, Maloney PC, Agre P (1999) J Mol Biol 291:1169–1179
Pohl P (2004) Biol Chem 385:921–926
Vila JA, Arnautova YA, Vorobjev Y, Scheraga HA (2011) Proc Natl Acad Sci USA 108:5602–5607
Cheng F, Sun H, Zhang Y, Mukkamala D, Oldfield E (2005) J Am Chem Soc 127:12544–12554
Roberts JD (2000) ABCs of FT-NMR, Sausalito, CA: University Science Books 258–259
Hu F, Luo W, Hong M (2010) Science 330:505–508
Lu YP, Yang CY, Wang SM (2006) J Am Chem Soc 128:11830–11839
Duan LL, Tong Y, Mei Y, Zhang QG, Zhang JZH (2007) J Chem Phys 127:145101–145106
Shi SH, Hu GD, Chen JZ, Zhang SL, Zhang QG (2009) Acta Chim Sinica 67:2791–2797
Sotomayor M, Schulten K (2007) Science 316:1144–1148
Colizzi F, Perozzo R, Scapozza L, Recanatini M, Cavalli A (2010) J Am Chem Soc 132:7361–7371
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33–38, 27-28
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926–935
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) J Comput Chem 26:1781–1802
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) J Phys Chem B 102:3586–3616
Feller SE, Zhang YH, Pastor RW, Brooks BR (1995) J Chem Phys 103:4613–4621
Tuckerman M, Berne BJ, Martyna GJ (1992) J Chem Phys 97:1990–2001
Darden T, York D, Pedersen L (1993) J Chem Phys 98:10089–10092
Park S, Schulten K (2004) J Chem Phys 120:5946–5961
Isralewitz B, Baudry J, Gullingsrud J, Kosztin D, Schulten K (2001) J Mol Graph Model 19:13–25
Chen LY (2008) J Chem Phys 129:144113–144117
Chen LY, Bastien DA, Espejel HE (2010) Phys Chem Chem Phys 12:6579–6582
Hu G, Chen LY (2010) Biophys Chem 153:97–103
Chen LY (2011) Phys Chem Chem Phys 13:6176–6183
Hirano Y, Okimoto N, Kadohira I, Suematsu M, Yasuoka K, Yasui M (2010) Biophys J 98:1512–1519
Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MSP (1996) J Mol Graph 14:354–360
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 03, revision C.02, Gaussian Inc., Wallingford
Acknowledgments
The authors acknowledge support from the National Institutes of Health (grant no. GM084834), the UTSA Computational Biology Initiative, and the Texas Advanced Computing Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, G., Chen, L.Y. & Wang, J. Insights into the mechanisms of the selectivity filter of Escherichia coli aquaporin Z. J Mol Model 18, 3731–3741 (2012). https://doi.org/10.1007/s00894-012-1379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1379-2